1. Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006; 107(4):927–941.
2. Iyasu S, Saftlas AK, Rowley DL, Koonin LM, Lawson HW, Atrash HK. The epidemiology of placenta previa in the United States, 1979 through 1987. Am J Obstet Gynecol. 1993; 168(5):1424–1429.

3. Ananth CV, Smulian JC, Vintzileos AM. The association of placenta previa with history of cesarean delivery and abortion: a metaanalysis. Am J Obstet Gynecol. 1997; 177(5):1071–1078.

4. Ananth CV, Wilcox AJ, Savitz DA, Bowes WA Jr, Luther ER. Effect of maternal age and parity on the risk of uteroplacental bleeding disorders in pregnancy. Obstet Gynecol. 1996; 88(4 Pt 1):511–516.

5. Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjaerven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006; 21(9):2353–2358.

6. Giudice LC, Kao LC. Endometriosis. Lancet. 2004; 364(9447):1789–1799.

7. Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011; 96(2):360–365.

8. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011; 17(3):327–346.

9. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17(5):637–653.

10. Healy DL, Breheny S, Halliday J, Jaques A, Rushford D, Garrett C, et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod. 2010; 25(1):265–274.

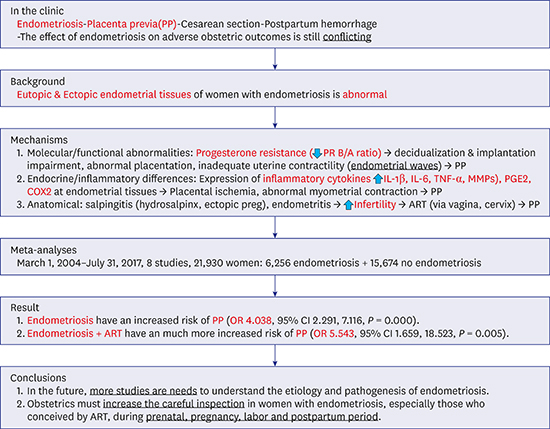
11. Takemura Y, Osuga Y, Fujimoto A, Oi N, Tsutsumi R, Koizumi M, et al. Increased risk of placenta previa is associated with endometriosis and tubal factor infertility in assisted reproductive technology pregnancy. Gynecol Endocrinol. 2013; 29(2):113–115.

12. Lin H, Leng JH, Liu JT, Lang JH. Obstetric outcomes in Chinese women with endometriosis: a retrospective cohort study. Chin Med J (Engl). 2015; 128(4):455–458.
13. Fujii T, Wada-Hiraike O, Nagamatsu T, Harada M, Hirata T, Koga K, et al. Assisted reproductive technology pregnancy complications are significantly associated with endometriosis severity before conception: a retrospective cohort study. Reprod Biol Endocrinol. 2016; 14(1):73–77.

14. Benaglia L, Candotti G, Papaleo E, Pagliardini L, Leonardi M, Reschini M, et al. Pregnancy outcome in women with endometriosis achieving pregnancy with IVF. Hum Reprod. 2016; 31(12):2730–2736.

15. Li H, Zhu HL, Chang XH, Li Y, Wang Y, Guan J, et al. Effects of previous laparoscopic surgical diagnosis of endometriosis on pregnancy outcomes. Chin Med J (Engl). 2017; 130(4):428–433.

16. Saraswat L, Ayansina DT, Cooper KG, Bhattacharya S, Miligkos D, Horne AW, et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG. 2017; 124(3):444–452.

17. Berlac JF, Hartwell D, Skovlund CW, Langhoff-Roos J, Lidegaard Ø. Endometriosis increases the risk of obstetrical and neonatal complications. Acta Obstet Gynecol Scand. 2017; 96(6):751–760.

18. Kim SG, Seo HG, Kim YS. Primiparous singleton women with endometriosis have an increased risk of preterm birth: meta-analyses. Obstet Gynecol Sci. 2017; 60(3):283–288.

19. Jeon YE, Cho S, Lee KE, Yang HI, Seo SK, Kim HY, et al. Prognostic factors for predicting spontaneous pregnancy after laparoscopic surgical treatment of endometriosis. Korean J Obstet Gynecol. 2009; 52(12):1287–1295.
20. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012; 18(12):1754–1767.

21. Gylfason JT, Kristjansson KA, Sverrisdottir G, Jonsdottir K, Rafnsson V, Geirsson RT. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am J Epidemiol. 2010; 172(3):237–243.

22. Chae U, Min JY, Kim SH, Ihm HJ, Oh YS, Park SY, et al. Decreased progesterone receptor B/A ratio in endometrial cells by tumor necrosis factor-alpha and peritoneal fluid from patients with endometriosis. Yonsei Med J. 2016; 57(6):1468–1474.

23. Zanatta A, Pereira RM, Rocha AM, Cogliati B, Baracat EC, Taylor HS, et al. The relationship among HOXA10, estrogen receptor α, progesterone receptor, and progesterone receptor B proteins in rectosigmoid endometriosis: a tissue microarray study. Reprod Sci. 2015; 22(1):31–37.
24. Yang SH, Kim HO, Sung NY, Ahn HS, Choo YS, Yang KM, et al. In vitro fertilization outcomes in patients with advanced endometriosis after surgical treatment. Korean J Obstet Gynecol. 2011; 54(9):517–522.
25. Vercellini P, Parazzini F, Pietropaolo G, Cipriani S, Frattaruolo MP, Fedele L. Pregnancy outcome in women with peritoneal, ovarian and rectovaginal endometriosis: a retrospective cohort study. BJOG. 2012; 119(12):1538–1543.

26. Kortelahti M, Anttila MA, Hippeläinen MI, Heinonen ST. Obstetric outcome in women with endometriosis--a matched case-control study. Gynecol Obstet Invest. 2003; 56(4):207–212.
27. Kuivasaari-Pirinen P, Raatikainen K, Hippeläinen M, Heinonen S. Adverse outcomes of IVF/ICSI pregnancies vary depending on aetiology of infertility. ISRN Obstet Gynecol. 2012; 2012:451915.

28. Smithers PR, Halliday J, Hale L, Talbot JM, Breheny S, Healy D. High frequency of cesarean section, antepartum hemorrhage, placenta previa, and preterm delivery in in-vitro fertilization twin pregnancies. Fertil Steril. 2003; 80(3):666–668.

29. Haas D, Wurm P, Shamiyeh A, Shebl O, Chvatal R, Oppelt P. Efficacy of the revised Enzian classification: a retrospective analysis. Does the revised Enzian classification solve the problem of duplicate classification in rASRM and Enzian? Arch Gynecol Obstet. 2013; 287(5):941–945.

30. Conti N, Cevenini G, Vannuccini S, Orlandini C, Valensise H, Gervasi MT, et al. Women with endometriosis at first pregnancy have an increased risk of adverse obstetric outcome. J Matern Fetal Neonatal Med. 2015; 28(15):1795–1798.









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download