1. National Research Council. Health Effects of Exposure to Radon: BEIR VI. Washington, D.C.: National Academies Press;1999.
2. Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol. 2001; 12(10):1341–1351.

3. Environmental Protection Agency. EPA Assessment of Risks from Radon in Homes (EPA 402-R-03-003). Washington, D.C.: Environmental Protection Agency;2003.
4. Pavia M, Bianco A, Pileggi C, Angelillo IF. Meta-analysis of residential exposure to radon gas and lung cancer. Bull World Health Organ. 2003; 81(10):732–738.
5. Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology. 2005; 16(2):137–145.
6. International Agency for Research on Cancer. Man-made Mineral Fibres and Radon. 1988.
7. National Toxicology Program. Seventh Annual Report on Carcinogens. Research Triangle Park, NC: National Institute of Environmental Health Sciences;1994.
8. World Health Organization. WHO Handbook on Indoor Radon: a Public Health Perspective. Geneva: World Health Organization;2009.
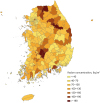
9. Cho BW, Choo CO, Kim MS, Hwang J, Yun U, Lee S. Spatial relationships between radon and topographical, geological, and geochemical factors and their relevance in all of South Korea. Environ Earth Sci. 2015; 74(6):5155–5168.

10. Appleton JD, Miles JC. A statistical evaluation of the geogenic controls on indoor radon concentrations and radon risk. J Environ Radioact. 2010; 101(10):799–803.

11. McColl N, Auvinen A, Kesminiene A, Espina C, Erdmann F, de Vries E, et al. European Code against Cancer 4th Edition: ionising and non-ionising radiation and cancer. Cancer Epidemiol. 2015. 39:Suppl 1. p. S93–S100.

12. Kim Y, Chang BU, Park HM, Kim CK, Tokonami S. National radon survey in Korea. Radiat Prot Dosimetry. 2011; 146(1-3):6–10.

13. Catelinois O, Rogel A, Laurier D, Billon S, Hemon D, Verger P, et al. Lung cancer attributable to indoor radon exposure in France: impact of the risk models and uncertainty analysis. Environ Health Perspect. 2006; 114(9):1361–1366.

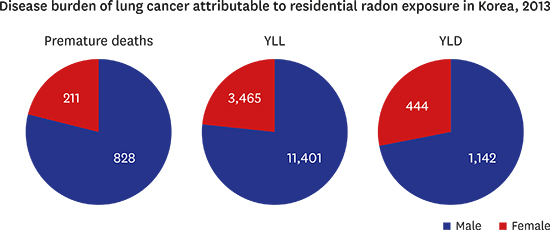
14. Zahra A, Cheong HK, Park JH. Burden of disease attributable to smoking in Korea. Asia Pac J Public Health. 2017; 29(1):47–59.

15. National Institute of Environmental Research. Nationwide Survey (2011–2012) of Indoor Radon at Home in Korea. Incheon: National Institute of Environmental Research;2012.
16. National Institute of Environmental Research. Nationwide Survey (2013–2014) of Indoor Radon at Home in Korea. Incheon: National Institute of Environmental Research;2014.
17. National Institute of Environmental Research. Nationwide Survey of Indoor Radon in Korea - Public Facilities. Incheon: National Institute of Environmental Research;2010.
18. Yoo JH, Seo SY, Jung JS, Lee KS, Lee JW, Kwon MH. Comparison of indoor radon concentrations by seasonal in some areas of Gangwondo. J Odor Indoor Environ. 2016; 15(3):204–212.

19. Ha M, Hwang SS, Kang S, Park NW, Chang BU, Kim Y. Geographical correlations between indoor radon concentration and risks of lung cancer, non-Hodgkin's lymphoma, and leukemia during 1999–2008 in Korea. Int J Environ Res Public Health. 2017; 14(4):E344.

20. Health Protection Agency. Radon and Public Health. Report of the Independent Advisory Group on Ionising Radiation. Documents of the Health Protection Agency. Radiation, Chemical and Environmental Hazards, RCE-11. Didcot: Health Protection Agency;2009.
21. Darby S, Hill D, Deo H, Auvinen A, Barros-Dios JM, Baysson H, et al. Residential radon and lung cancer--detailed results of a collaborative analysis of individual data on 7148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health. 2006; 32:Suppl 1. 1–83.
22. Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015; 3(11):e712–e723.

23. World Health Organization. WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2011. Geneva: World Health Organization;2013.
24. Jung KW, Won YJ, Kong HJ, Oh CM, Shin A, Lee JS. Survival of Korean adult cancer patients by stage at diagnosis, 2006–2010: national cancer registry study. Cancer Res Treat. 2013; 45(3):162–171.

25. Jeong YJ, Kim DY. Cases and literature review of timing for withdrawal of palliative chemotherapy. Korean J Hosp Palliat Care. 2016; 19(1):70–75.

26. Pawel DJ, Puskin JS. The U.S. Environmental Protection Agency's assessment of risks from indoor radon. Health Phys. 2004; 87(1):68–74.

27. Cao X, MacNaughton P, Laurent JC, Allen JG. Radon-induced lung cancer deaths may be overestimated due to failure to account for confounding by exposure to diesel engine exhaust in BEIR VI miner studies. PLoS One. 2017; 12(9):e0184298.

28. Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005; 330(7485):223.

29. Heid IM, Küchenhoff H, Rosario AS, Kreienbrock L, Wichmann HE. Impact of measurement error in exposures in German radon studies. J Toxicol Environ Health A. 2006; 69(7):701–721.

30. Xuan XZ, Lubin JH, Li JY, Yang LF, Luo AS, Lan Y, et al. A cohort study in southern China of tin miners exposed to radon and radon decay products. Health Phys. 1993; 64(2):120–131.
32. Jeon JS, Lee JY, Eom SW, Chae YZ. The variation characteristics of indoor radon concentration from buildings with different environment, Seoul. J Korean Soc Atmos Environ. 2011; 27(6):692–702.

33. Lee K, Seo S, Yoo J, Oh S, Kwon M, Lee W. Factors influencing indoor radon concentration in detached houses. J Odor Indoor Environ. 2016; 15(2):93–99.

34. Lee C, Gwak Y, Lee D, Lee D, Cho Y. A study on the prediction of indoor concentration due to radon exhalation from domestic building materials. J Environ Sci Int. 2015; 24(9):1131–1138.

35. Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, et al. Prognosis in advanced lung cancer--a prospective study examining key clinicopathological factors. Lung Cancer. 2015; 88(3):304–309.
36. Choi Y, Keam B, Kim TM, Lee SH, Kim DW, Heo DS. Cancer treatment near the end-of-life becomes more aggressive: changes in trend during 10 years at a single institute. Cancer Res Treat. 2015; 47(4):555–563.

37. Krstić G. Radon versus other lung cancer risk factors: How accurate are the attribution estimates? J Air Waste Manag Assoc. 2017; 67(3):261–266.

38. Lee HA, Lee WK, Lim D, Park SH, Baik SJ, Kong KA, et al. Risks of lung cancer due to radon exposure among the regions of Korea. J Korean Med Sci. 2015; 30(5):542–548.

39. Menzler S, Piller G, Gruson M, Rosario AS, Wichmann HE, Kreienbrock L. Population attributable fraction for lung cancer due to residential radon in Switzerland and Germany. Health Phys. 2008; 95(2):179–189.

40. Peterson E, Aker A, Kim J, Li Y, Brand K, Copes R. Lung cancer risk from radon in Ontario, Canada: how many lung cancers can we prevent? Cancer Causes Control. 2013; 24(11):2013–2020.

41. GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390(10100):1345–1422.
43. Shin A, Oh CM, Kim BW, Woo H, Won YJ, Lee JS. Lung cancer epidemiology in Korea. Cancer Res Treat. 2017; 49(3):616–626.

46. Lantz PM, Mendez D, Philbert MA. Radon, smoking, and lung cancer: the need to refocus radon control policy. Am J Public Health. 2013; 103(3):443–447.

47. Kim I, Bahk J, Yoon TH, Yun SC, Khang YH. Income differences in smoking prevalences in 245 districts of South Korea: patterns by area deprivation and urbanity, 2008–2014. J Prev Med Public Health. 2017; 50(2):100–126.

48. Zoo DH, Park KH, Jeong HW, Lim HJ, Bok DS, Yun DW, et al. A study on indoor radon concentration among vulnerable households in Korea. J Environ Health Sci. 2015; 41(2):61–70.










 PDF
PDF Citation
Citation Print
Print



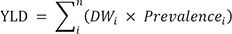
 XML Download
XML Download