Abstract
Background
The purpose of this study was to evaluate the usefulness of sonication technique for microbiological diagnosis and the sterility of the recycled autoclaved femoral components from infected total knee arthroplasty (TKA) using a sonication method.
Methods
Nineteen femoral implants explanted from patients with infected TKA were sterilized with a standard autoclave method. Standard culture of the fluid before and after sonication of the sterilized implants was performed to detect pathogenic microorganisms. Additional experiments were performed to evaluate the sterility of the recycled implant by inducing artificial biofilm formation. Methicillin-resistant Staphylococcus aureus (MRSA) was inoculated into 10 implants and sterilization in a standard autoclave was performed, and then the fluid was cultured before and after sonication.
Results
Two of the 19 sterilized implants were positive for growth of bacteria after sonication, whereas no growth was detected in the cultured fluid from the sterilized implants before sonication. The bacteria were Staphylococcus species in all two cases. In one of 10 implants inoculated with MRSA, the culture was positive for growth of bacteria both before and after sonication. However, Staphylococcus epidermidis was cultured from both occasions and thus this implant was thought to be contaminated.
Conclusions
We found sonication for identification of pathogens could be helpful, but this finding should be interpreted carefully because of the possibility of contamination. Sterilization of an infected femoral implant with an autoclave method could be a good method for using the temporary articulating antibiotic spacer in two-stage revision arthroplasty.
The incidence of total knee arthroplasty (TKA) and revision TKA for treatment failure has been increasing. Infection is one of the most common complications requiring revision after TKA.1) A recent epidemiologic study reported that the incidence and prevalence of periprosthetic joint infection may be increasing over time in the United States.2) The incidence of infection was reported to be 1%–2% in primary TKA and 4%–8% in revision TKA.3)
In patients with a suspected infection, there are cases without culture of pathogens despite definite symptoms or signs of infection. Moreover, there are cases in which the culture of pathogens from the specimen during the operation is positive whereas no pathogen was cultured before the operation. Therefore, diagnosis of infection and identification of the pathogens are very important for treatment.
The appropriate antibiotic therapy must be initiated until second-stage revision arthroplasty. Therefore, identification of the pathogens is important for the treatment of infection. Chronic periprosthetic infections are related to tolerance to the antibiotics and defense mechanisms of the host and biofilm formation. In particular, Staphylococcus aureus, which is one of the most common pathogens of postoperative infection,4) can attach to the surface of implants and form a biofilm that may not be identified by traditional microbiological techniques.5) The identification of pathogens and appropriate treatment are difficult in this situation.
Therefore, many methods for the identification of pathogens have been attempted. Most surgeons use a culture of the periprosthetic tissue from infected implants for the identification of the pathogen. Peel et al.6) reported a sensitivity of 92% after 1 day with periprosthetic tissues using a blood culture bottle. Recently, sonication of infected implants to remove the biofilm followed by culture has been shown to be a more sensitive and accurate method for the identification of pathogens by several studies.789)
In cases of chronic infection after TKA, a two-stage
revision arthroplasty has been recommended as a treatment. In the first stage of revision arthroplasty, culture of the pathogenic bacteria using the removed implant and periprosthetic tissue is necessary for the identification of the pathogen. An articulating spacer molded by antibioticmixed bone cement is used,1011) or the autoclaved femoral component removed from the patients is reused in this stage. These methods are both widely used and good clinical results have been reported by several authors.121314) Therefore, the sterility of recycled femoral components is important for successful operation. Lyons et al.14) reported on the sterility of the autoclaved implant and found the autoclaved femoral component could reduce the biofilm burden significantly.
We hypothesized that the reuse of an autoclaved femoral implant from an infected TKA would be safe microbiologically and evaluated the results of culture from a sterilized implant using the autoclave method for patients with an infected TKA before and after sonication. The purpose of this study was to evaluate the usefulness of sonication technique for microbiological diagnosis and the sterility of the recycled autoclaved femoral components from TKA using a sonication method.
Nineteen infected femoral implants without identified pathogens were prepared. Sterilization of the implants with an autoclave sterilizer (Tomy SX-700; Seiko, Tokyo, Japan) was performed at 121℃ for 20 minutes after removal of periprosthetic tissues and cleansing were performed. The implants were then aseptically transferred into a stomacher bag (20 × 30 cm; BNF Korea, Gimpo, Korea). Four hundred milliliters of sterile saline was added to the stomacher bag with the implant in a laminar airflow biosafety cabinet, and then the stomacher was vortexed for 30 seconds (Fig. 1). Thirty milliliters of sterile saline was taken from the stomacher bag and centrifuged at 6,000 rpm for 5 minutes. All but 1 ml of the supernatant was decanted. The centrifuge tube was vortexed until the precipitate was resuspended completely and 100 µL each was spread onto four different selective agar plates: macConkey agar plate (MAC) for Gram-negative organism, sheep blood agar plate (BAP) for fastidious organism and detecting hemolytic activity, chocolate agar plate (CAP) for fastidious respiratory bacteria, and Centers for Disease Control and Prevention (CDC)-BAP for anaerobes. BAP, CAP, and MAC were incubated aerobically at 37℃ for 4 days and CDC was incubated anaerobically at 37℃ for 7 days. Bacterial colonies on the agar media were observed phenotypically and identified using an API biochemical kit (Biomerieux, Marcy-l'Etoile, France). A culture was considered positive if one colony per plate (1 colony forming unit [CFU]/plate) was counted.
The stomacher bag with the 400 mL of saline and implant was subjected to sonication (Branson 3510; Branson Ultrasonic, Danbury, CT, USA) for 5 minutes (40 kHz, 185 W) and vortexed for 30 seconds (Fig. 2). The same process for culture was then repeated as described above.
The previous pathogens before autoclave sterilization were unknown in the previous experiment. Therefore, an additional experiment was performed using the implant with direct inoculation of methicillin-resistant Staphylococcus aureus (MRSA).
A total of 10 implants were sterilized with an autoclave (Tomy SX-700, Seiko) for 20 minutes at 121℃. The pathogen, MRSA (strain NCTC10442), was incubated in brain heart infusion medium. Four hundred microliters were inoculated into the stomacher bag with 400 mL of sterile saline and the sterilized implant. The infected implant was incubated aerobically at 37℃ for 3 days. After that, cultures after sonication were performed as described above. MAC, BAP, CAP, and mannitol salt agar were used for culture media. A culture was considered positive if one colony per plate (1 CFU/plate) was counted.
In the first experiment, no implant showed a positive bacterial culture before sonication; however, two implants showed positive bacterial cultures after sonication (Table 1). Three colonies were cultured from each implant. Two colonies on BAP and one colony on CDC were in one implant and two colonies on CAP and one colony on BAP were in the other implant. Biochemical identification using an API staph kit was performed. One implant had two colonies of Staphylococcus hominis and one colony of Staphylococcus capitis. The other implant had three colonies of Staphylococcus species. (Table 2).
In the additional experiment with MRSA inoculation of 10 sterilized implants, one implant showed positive bacterial culture in BAP before sonication and another implant showed positive bacterial culture in BAP after sonication. Staphylococcus epidermidis was identified from both implants (Table 3). MRSA used for direct inoculation was not cultured in this experiment. The cultured pathogen was intended to represent contamination during the process of sonication.
In this study, the first experiment showed the higher rate of detection of bacterial cultures for sonication and the incompleteness of sterilization of the autoclaved prosthesis. However, in the experiment using directly inoculated implants, the cultured pathogens were not those we inoculated, indicating contamination. Therefore, in the first experiment, the possibility of contamination with pathogens after autoclaving should not be entirely excluded as well as the possibility of survival of pathogens.
The difficulty in treatment of infected TKA is related to resistance to antibiotics, host defense mechanisms, and biofilm formation.5) Biofilm formation is initiated with adherence of bacteria to the implant.15) Biofilms consist of microorganisms enveloped by macromolecules of glycocalyx and other protective films.16) The chemical bonding of bacterial extracapsular structures to the surface of implants can result in microbial adhesion. Bacteria with biofilm formation cannot be cultured due to adhesion to the implant,17) and treatment with traditional concentrations of antibiotics is ineffective for eradication of the biofilm population.181920)
Therefore, biofilm detachment from the implant using sonication and culture of the fluid could increase the sensitivity of diagnosis.8) Tunney et al.21) and Trampuz et al.8) reported that the accuracy of diagnosis was improved by sonication and appropriate antibiotic therapy was possible for infected TKA. The sensitivity of culture for an infected TKA is decreased when antibiotics therapy is initiated before removal of the implant.8) Many surgeons start antibiotic therapy before surgery for implant removal, and in these cases, sonication could be useful to detect the pathogens. In the first experiment, the bacteria were cultured after sonication from the autoclave-sterilized implants whereas no growth was obtained before sonication. This showed the higher efficacy of sonication for identification of pathogens.
Contamination during the process of experiment is possible. Trampuz et al.22) reported that sonication had a lower sensitivity because of leakage from the bag despite its high specificity. In this study, coagulase-negative Staphylococci were cultured in the first experiment. This is one of the most common pathogens of periprosthetic infection,2324) and therefore this could be supposed to be the pathogen for TKA infection rather than the result of contamination. However, implants subjected to autoclave sterilization twice were used in the additional experiment using inoculation of MRSA. The inoculated MRSA was not cultured in the implants, but S. epidermidis was cultured, indicating the possibility of contamination. Therefore, it is difficult to rule out the possibility of contamination with the cultured bacteria in the first experiment although the possibility of them being the real pathogens also exists.
The protocol of sonication should also be considered. Various sonication methods were used in several previous studies and the results of culture could be affected by the different sonication methods. Some studies reported time and temperature during sonication could affect the culture results.25) Monsen et el.25) reported a sonication method used at 22℃ for 7 minutes (40 kHz, 350 W) and another method at room temperature for 5 minutes (40 kHz, 185 W) could affect the results of the culture. Therefore, multiple factors that could influence the culture results should be evaluated.
Two-stage revision arthroplasty is mostly used for patients with a periprosthetic joint infection. In the firststage of the revision arthroplasty, reuse of the sterilized explanted femoral implant for the articulating spacer can reduce the cost and the inflammatory reaction by the cement particles on the friction surface when using the cement-cement molded spacer. However, Lyons et al.14) reported the autoclave could not thoroughly eradicate biofilms on the surface of the implant, although the biofilm burden could be reduced significantly. In the current study, the first experiment showed autoclave sterilization could not eradicate all bacteria of the infected implant. However, the possibility of contamination during the process should not be excluded. Moreover, we could not determine whether the positive culture results (1 CFU/plate) in this study were clinically meaningful. Satisfactory clinical results, based on reports by several authors, could be obtained using weakness of bacteria by autoclaving and a combination of antibiotics-mixed bone cement along with intravenous antibiotics therapy.132627) Therefore, reusing the autoclaved implant could be an effective method for using the temporary articulating spacer if the concomitant antibiotic treatment for the infection should be combined thoroughly.
There are limitations of this study. Only bacterial cultures were performed; other pathogens, including viruses and fungi, could remain after autoclave sterilization. Additional studies detecting other pathogens should be performed. The possibility of contamination of the stomacher bag during the process of experiment could not be excluded. The protocol of sonication should also have been considered. In this study, sonication (40 kHz, 185 W) performed at room temperature for 5 minutes was different from that in other studies and could have affected the results of the culture. Moreover, three days of incubation with MRSA could have been an insufficient time for biofilm formation. However, Lyons et al.14) confirmed biofilm formation after only 1 day, and therefore we presumed 3 days would be sufficient. finally, one colony per plate was defined as a positive culture in this study, but it is not clear whether this definition has any clinical significance. Several studies have used variable criteria for positive culture results: Scorzolini et al.7) used 5 CFU/mL in a 0.5 mL inoculation; Trampuz et al.8) used 5 CFU/plate in a 0.5 mL inoculation; and Zalavras28) used 20 CFU/mL. Definite criteria have not been established. Therefore, these criteria should also be evaluated in further studies. Finally, preoperative culture of the femoral component was not evaluated in this study; therefore, we could not know whether the cultured bacteria after sonication of the autoclaved femoral component were the pathogens that caused periprosthetic joint infection.
In this study, sonication for identification of pathogens could be helpful showing a higher detection rate of pathogens; however, this should be interpreted carefully because of the possibility of contamination. In addition, sterilization of an infected femoral implant with an autoclave method could be a good method for using the temporary articulating antibiotic spacer in two-stage revision arthroplasty.
Figures and Tables
 | Fig. 1The implants were aseptically transferred into a stomacher bag and 400 mL of sterile saline was added to the stomacher bag. |
Table 1
Results of Bacterial Culture of the Implants before and after Sonication
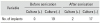
| Variable | Before sonication | After sonication | ||
|---|---|---|---|---|
| Culture (+) | Culture (−) | Culture (+) | Culture (−) | |
| No. of implants | 0 | 19 | 2 | 17 |
Table 2
Biochemical Identification Results of the Cultured Bacteria from the Implants

References
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010; 468(1):45–51.

2. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008; 23(7):984–991.

3. Kyung HS, Mun JU. Treatment of infections after total knee arthroplasty. J Korean Orthop Assoc. 2010; 45(5):335–341.

4. Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013; 56(2):182–194.
5. Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002; 292(2):107–113.

6. Peel TN, Dylla BL, Hughes JG, et al. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. MBio. 2016; 7(1):e01776-15.

7. Scorzolini L, Lichtner M, Iannetta M, et al. Sonication technique improves microbiological diagnosis in patients treated with antibiotics before surgery for prosthetic joint infections. New Microbiol. 2014; 37(3):321–328.
8. Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007; 357(7):654–663.

9. Mariconda M, Ascione T, Balato G, et al. Sonication of antibiotic-loaded cement spacers in a two-stage revision protocol for infected joint arthroplasty. BMC Musculoskelet Disord. 2013; 14:193.

10. Shaikh AA, Ha CW, Park YG, Park YB. Two-stage approach to primary TKA in infected arthritic knees using intraoperatively molded articulating cement spacers. Clin Orthop Relat Res. 2014; 472(7):2201–2207.

11. Ha CW. A technique for intraoperative construction of antibiotic spacers. Clin Orthop Relat Res. 2006; 445:204–209.

12. Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995; (321):45–54.

13. Kim SH, Han HS, Kim DH, Kang SB. Two-stage reimplantation in infected total knee arthroplasty (a method of reinsertion of the autoclaved femoral component and a polyethylene liner). J Korean Knee Soc. 2010; 22(2):110–116.
14. Lyons ST, Wright CA, Krute CN, Rivera FE, Carroll RK, Shaw LN. Confirming sterility of an autoclaved infected femoral component for use in an articulated antibiotic knee spacer: a pilot study. J Arthroplasty. 2016; 31(1):245–249.

15. Gristina AG, Naylor PT, Myrvik QN. Mechanisms of musculoskeletal sepsis. Orthop Clin North Am. 1991; 22(3):363–371.

16. Akiyama H, Hamada T, Huh WK, et al. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br J Dermatol. 2003; 148(3):526–532.

17. Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue: the significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985; 67(2):264–273.

18. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995; 49:711–745.

19. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999; 37(6):1771–1776.

20. Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002; 66(2):86–92.
21. Tunney MM, Patrick S, Gorman SP, et al. Improved detection of infection in hip replacements: a currently underestimated problem. J Bone Joint Surg Br. 1998; 80(4):568–572.
22. Trampuz A, Piper KE, Hanssen AD, et al. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol. 2006; 44(2):628–631.

23. Song Z, Borgwardt L, Hoiby N, Wu H, Sorensen TS, Borgwardt A. Prosthesis infections after orthopedic joint replacement: the possible role of bacterial biofilms. Orthop Rev (Pavia). 2013; 5(2):65–71.

24. Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010; 34(4):505–510.

25. Monsen T, Lovgren E, Widerstrom M, Wallinder L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J Clin Microbiol. 2009; 47(8):2496–2501.

26. Lee BJ, Kyung HS, Yoon SD. Two-stage revision for infected total knee arthroplasty: based on autoclaving the recycled femoral component and intraoperative molding using antibiotic-impregnated cement on the tibial side. Clin Orthop Surg. 2015; 7(3):310–317.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download