Abstract
Background
We aimed to examine the factors that influence synovialization of the grafted tendon after double-bundle anterior cruciate ligament (ACL) reconstruction based on second-look arthroscopic findings.
Methods
Out of 205 knees that were treated between August 2008 and May 2016 with double-bundle ACL reconstruction using bio-absorbable cross-pins and Endobuttons for femoral tunnel fixation, we enrolled 65 knees (64 patients) that underwent second-look arthroscopy with hardware removal at 1 year postoperatively. Measured clinical outcomes included the Lysholm score and Tegner activity score that were evaluated preoperatively and during the final follow-up. We analyzed the relationship between synovial coverage and patient age, length of the preserved remnant tissue on the tibial side, type of bundle (anteromedial or posterolateral), type of graft (autograft or allograft), and time from injury to surgery.
Results
The area of synovial coverage showed a significant statistical correlation with patient age and the length of the preserved remnant tissue on the tibial side. The average synovial coverage was significantly better for the anteromedial bundle than for the posterolateral bundle, better for the autograft than for the allograft reconstruction, and better when treated in the acute stage than in the chronic stage. However, synovialization of grafted tendon did not correlate to clinical outcomes.
Conclusions
While we were able to identify several factors influencing synovialization of the grafted tendon after double-bundle ACL reconstruction, including patient age, length of preserved remnant tissue of the torn ACL, type of bundle, type of graft, and time from injury to surgery, we found no evidence that increased synovialization improves clinical outcomes at 1 year postoperatively.
In the presence of anterior instability in the knee joint, it is difficult to create the femoral tunnel in its anatomical position during single-bundle reconstruction using the transtibial technique, which may result in rotational instability of the treated knee. To address this concern, surgeons have attempted double-bundle reconstruction in an effort to create the femoral tunnel in its anatomical position.1234) However, even if the grafted tendon is mechanically stabilized using a double-bundle technique, inadequate biological healing of the grafted tendon eventually leads to fibrillation due to mechanical irritation over a long duration, thereby inducing weakening and rerupture of the grafted tendon. Postoperative synovialization of the grafted tendon after anterior cruciate ligament (ACL) reconstruction is known to help maintain the proprioceptive sense as well as prevent weakening and rerupture of the graft tendon through revascularization of the surface.25) In addition, preservation of the remnant tissue of the tibial side of the torn ACL helps preserve the remaining mechanoreceptors. Therefore, recent techniques apply various approaches to preserve the remnant ACL tissue as much as possible during surgical treatment.3678) In the present study, we examined the factors that may have a role in the synovialization of the grafted tendons after double-bundle ACL reconstruction using an autologous hamstring tendon and a tibialis anterior allograft tendon. To this end, patient age, length of the remnant tissue on the tibial side, type of graft tendon (autograft vs. allograft), type of graft bundle (anteromedial bundle vs. posterolateral bundle), and time from injury to surgery were analyzed in relation to the extent of synovialization, as indicated by second-look arthroscopy at 1 year after ACL reconstruction.
Protocol approval was obtained from the Institutional Review Board of Pohang St. Mary's Hospital (IRB No. 0749-170519-HR-029-01). The study was performed in accordance with the ethical standards established in the1964 Declaration of Helsinki and its later amendments. The informed consent was waved.
A total of 205 knees with a rupture of the ACL were treated in our hospital with double-bundle reconstruction between August 2008 and May 2016. The present study included only patients older than 16 years of age after skeletal maturation around the knee joint and patients who wanted to do sport activities after surgery. At least 1 year after operation, patients who had pain, tenderness, or discomfort on the tibial screw site under the skin were selected. We performed metal screw removal and second-look arthroscopy to inspect the status of the grafted ACL. Patients were excluded if they had revision ACL reconstruction. We selected 65 knees from 64 patients. Depending on the time from injury to surgery, the operations were classified as performed in the acute stage (< 3 months from injury, 47 knees) or in the chronic stage (≥ 3 months after injury, 18 knees).
Two tunnels were created in the femur and a single tunnel was created in the tibia to enable double-bundle ACL reconstruction. The double bundle consisted of an autologous hamstring tendon (four strands of the gracilis and semitendinosus tendons) and two strands of the tibialis anterior allograft tendon. Anteromedial bundle with four-strand autologous hamstring graft and posterolateral bundle with tibialis anterior allograft were used in 39 cases, and anteromedial bundle with allograft and posterolateral bundle with autograft were used in 26 cases. Knees that required two allografts (anteromedial bundle and posterolateral bundle) because of severe damage to the medial collateral ligament or difficulty in harvesting the autologous hamstring tendon due to scar adhesion at pes anserinus were excluded from this study.
The tibial tunnel was placed at the center of the tibial attachment of the ACL. The anteromedial femoral tunnel was created at an “11 o'clock” or “1 o'clock” position from the posterior ridge of the intercondylar notch of the lateral femoral condyle using a transtibial femoral tunnel guide, and the posterolateral femoral tunnel was created at the center of the anatomical attachment of the posterolateral bundle via an outside-in technique.
The tibial tunnel was initially created at a smaller diameter than the required size and was gradually enlarged using a dilator to a diameter of 8 mm to 10 mm with an interval of 0.5 mm, through which the graft tendon barely passed through, leaving no space for the joint fluid to leak into the tunnel. The anteromedial femoral tunnel was initially created to have a diameter of 8 mm and it was gradually enlarged using a dilator as in the same technique for the tibial tunnel; the posterolateral femoral tunnel was created to have a diameter of 6 mm from the cortex of the lateral condyle of the femur to the medial wall of the lateral femoral condyle.
The anteromedial femoral tunnel was created through a transtibial tunnel. Bioabsorbable cross-pins (RigidFix; DePuy Mitek, Raynham, MA, USA) were used for femoral side fixation of the anteromedial bundle, and Endobutton (Smith & Nephew, Memphis, TN, USA) was used for fixation of the posterolateral bundle. One tunnel was created at the tibia, and the anteromedial and posterolateral bundles were placed in the front and in the back of the tibial tunnel, respectively. The bundles were fixed at the tibial tunnel with a bioabsorbable interference screw. To prevent weakening of the tibial fixation, an additional anchor screw was applied in the distal portion of the tibial tunnel using a post-tie suture. The remnant tissue at the tibial attachment of the ACL was preserved as much as possible and the length of remnant tissue at the tibial side was measured by an arthroscopic ruler.
At least 1 year after ACL reconstruction, second-look arthroscopy was performed while removing the anchor screw used to fix the graft tendon on the tibial side. Based on the findings of second-look arthroscopy, we assessed the extent of synovial coverage in the grafted tendon. To ensure consistency of the measurements, the same investigator, who is a surgeon, assessed the extent of synovial coverage in all knees included in our study. Both the anteromedial and posterolateral bundles of the grafted ACL were formally divided into three parts (proximal, middle, and distal thirds), and the synovial coverage in each of the three parts was calculated as a percentage of the total bundle area. The mean synovial coverage was assessed for each third of the anteromedial and posterolateral bundles. The overall mean synovial coverage was calculated.
To compare the areas of synovial coverage more accurately, the anterior, posterior, medial and lateral sides were observed carefully, and a 70° arthroscope was used in areas with limited visibility. In addition, in vague cases, a synovial sample was taken for biopsy. The Lysholm score and Tegner activity score were assessed preoperatively and at the final follow-up for a functional assessment of the knee joint. The pivot shift test was performed preoperatively and at the final follow-up to assess the stability of the knee joint.
Finally, the correlation between the degree of synovial regeneration and several parameters was analyzed. The evaluated parameters included: (1) patient's age, (2) length of the remnant tissue at the tibial attachment of the torn ACL at the time of operation, (3) type of bundle (anteromedial or posterolateral), (4) type of graft tendon (autograft or allograft), and (5) time from injury to surgery. All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA). Pearson correlation coefficient was used to assess the correlation of synovialization with age and length of remnant tissue, whereas a two-sample t-test was used to compare synovial coverage according to type of bundle (anteromedial bundle vs. posterolateral bundle), type of graft tendon (autograft vs. allograft), and time of surgery (acute stage vs. chronic stage). Preoperative and postoperative clinical scores (Lysholm and Tegner activity scores) and pivot shift test scores were compared via the Wilcoxon signed rank test. A p-value < 0.05 was assumed to indicate statistical significance.
Between 2008 and 2015, 71 patients underwent second-look arthroscopy and met the inclusion criteria, but seven patients were excluded from the study due to a history of revision ACL reconstruction. Of the 65 remaining knees in the 64 patients (mean age, 29.7 years; range, 16 to 52 years) included in the study, 48 cases involved male patients and 17 cases involved female patients.
Participating in sports was the most common cause of injury (n = 43), followed by fall from height (n = 13), other causes (n = 5), and direct trauma (n = 4). Forty-two cases involved a combined injury, with 20 cases involving a medial meniscus tear, 10 cases involving a lateral meniscus tear, and eight cases involving mild medial collateral ligament injury. Second-look arthroscopy after double-bundle ACL reconstruction revealed that synovial coverage of the anteromedial bundle was 77.2%, 76.8%, and 81.8% in the proximal, middle, and distal region, respectively, with a mean synovialization of 78.6% ± 18.7%. For the posterolateral bundle, synovial coverage was 66.9%, 61.6%, and 64.6% in the proximal, middle, and distal region, respectively, with a mean coverage of 64.3% ± 21.1%. For the entire reconstructed ACL, the mean synovialization was 71.5% ± 16.7% (Table 1).
There was a significant correlation between age and synovialization (Pearson correlation coefficient, 0.52; p = 0.003), where younger patients exhibited greater synovialization. There was also a significant correlation between synovialization and the length of the remnant tissue at the tibial attachment (Pearson correlation coefficient, 0.42; p = 0.038), as synovialization increased with the length of the remnant tissue. Furthermore, the two-sample t-test showed that synovialization was significantly more extensive for anteromedial bundles than for posterolateral bundles (78.6% vs. 64.3, p = 0.001) (Table 1 and Fig. 1). Additionally, synovialization was significantly more pronounced for autografts than for allografts (81.1% vs. 63.4%, respectively; p = 0.001) (Table 1 and Fig. 2). Furthermore, synovialization was significantly higher in ACLs treated during the acute stage (< 3 months of injury) than in ACLs treated during the chronic stage (≥ 3 months after injury) (76.5% vs. 58.6%; p = 0.005) (Table 1 and Fig. 3).
The Lysholm and Tegner activity scores improved from the preoperative values of 66.1 points and 6.5 points, respectively, to 88.9 points and 8.9 points, respectively, at the time of final follow-up (Table 2). Furthermore, the pivot shift test scores, which were +1 in seven knees, +2 in 35 knees, and +3 in 23 knees preoperatively, were markedly improved at the final follow-up, where 44 knees were negative, and only 18 and three knees exhibited a score of +1 and +2, respectively (Table 3). However, the Wilcoxon signed-rank test indicated that the clinical assessment scores—Lysholm score (p = 0.27), Tegner activity score (p = 0.38), and pivot shift test score (p = 0.21)—were not significantly statistically associated with synovialization.
Second-look arthroscopy confirmed the presence of cyclops lesion in one case, but without clinical findings of the limitation of extension. Additionally, hypertrophy of the distal attachment area of the grafted tendon was found in two cases, neither showing symptoms of flexion contracture in the joint. In all three cases, the hypertrophic masses were shrunk using a bipolar radiofrequency system (Arthrocare, Sunnyvale, CA, USA) during second-look arthroscopic surgery.
To prevent rerupture and facilitate stable engraftment of the grafted tendon after ACL reconstruction, adequate preservation of proprioception of the grafted tendon is essential. Furthermore, revascularization and synovialization must occur.2368) Since Schultz et al.9) first described on mechanoreceptors and proprioception in ACL, mechanoreceptors and nerve bundles have been known to exist in the remnant tissues after ACL rupture. Ochi et al.10) substantiated the existence of somatosensory-evoked potentials in the ACL remnant tissue, which suggests that there are residual normal mechanoreceptors in the remnant tissues after ACL rupture. Moreover, they confirmed that preserving the ACL remnant during reconstruction is conducive to the regeneration of nerve endings and recovery of proprioception in the graft tendon, even after 18 months of injury. Sherman et al.11) reported that acute ACL injury mostly occurs within half of the proximal area, while Schutte et al.12) reported that most mechanoreceptors are located at the tibial attachment sites. According to Lee et al.,13) preserving the remnant tissue in the tibial area maintains the function of mechanoreceptors and helps postoperative healing and functional recovery of the graft tendon. Ochi et al.14) described a minimally invasive method of ACL augmentation that involves maximal preservation of ACL remnant tissues to preserve mechanoreceptors within the remnant tissue, thereby stimulating revascularization and reinnervation of the graft tendon.
However, preserving the remnant tissue during ACL reconstruction is challenging because of the limited surgical field of view and difficulty in selecting an accurate position for the tibial tunnel. Techniques that preserve the remnant tissue have been suggested by multiple studies to stimulate revascularization of the graft tendon, which facilitate its ligamentization by not disturbing the remaining vasculature.81415) Lee et al.813) reported that maximally preserving the remnant tissue at the tibial attachment of the ruptured ACL and running the graft tendon through the center of the remnant tissue stimulated synovialization and vascularization, and that placing the graft tendon within the tibial attachment allowed it to adopt a shape similar to the original ACL shape, thereby preventing impingement against the intercondylar notch during extension and lowering the risk of cyclops lesion. In addition, it has been reported that preserving remnant tissue in the tibial attachment prevents the influx of joint fluid into the tibial tunnel (a suspected cause of postoperative tunnel widening) and subsequently reduces tunnel widening by achieving close contact between the remnant tissue and the graft tendon.81314) Fremerey et al.16) reported that athletes whose remnant tissues were preserved during ACL reconstruction showed superior outcomes in terms of functional stability, rehabilitation, and return to sports compared to the outcomes of athletes whose remnant tissue was not preserved.
In knees with a relatively large amount of remnant tissue at the tibial attachment site, we incised the remnant tissue longitudinally at the tibial attachment of the torn ACL with a #11 knife and placed the tibial tunnel at the center of the tibial attachment to preserve the remnant tissue as much as possible. Moreover, a cannulated reamer smaller than the diameter of the graft tendon was used to create the tibial tunnel, after which a dilator was used to enlarge the tibial and femoral tunnels to maximize the embedding of the graft tendon into the remnant tissue and prevent the influx of joint fluid into the tunnel. In knees with long attachment sites, where it was difficult to attach the graft tendon to the tibia, the remnant tissue was sutured to the grafted tendon to enhance healing of the graft tendon (Fig. 4). In fact, many knees treated in the acute stage (< 3 months of injury) had involved relatively longer remnant tissue, whereas knees treated in the chronic stage had relatively shorter remnant tissue that could be preserved, which was atrophied or presented as scar tissue. Furthermore, it was also shown that the length of preserved remnant tissue at the tibial attachment during ACL reconstruction was directly proportional to the extent of synovialization, but it was not correlated with mechanical stability of the ligament.17)
We found that, compared to the use of allografts, using autografts resulted in better synovialization in both the anteromedial and posterolateral bundles. In particular, the biopsies performed on samples taken from the surface of the ACL during second-look arthroscopy revealed that knees treated with autografts had thicker synovium and a more abundant amount of synovial lining cells (Fig. 5). Rice et al.18) also reported that ACL reconstruction with autografts led to superior outcomes compared to those obtained when using allografts, regardless of the age of the patient. In addition, Ahn et al.4) reported that second-look arthroscopic findings showed less synovialization in the posterolateral bundle than in the anteromedial bundle after double-bundle ACL reconstruction. Therefore, under the assumption that the posterolateral bundle is more likely to have a lower engraftment rate than the anteromedial bundle and that autografts would be more beneficial than allografts for synovialization, we ensured that the same number of autografts and allografts were used for the anteromedial and posterolateral bundles to minimize statistical error.
In our patients, none of the bundles (anteromedial or posterolateral) showed rupture on second-look arthroscopy; however, the anteromedial bundles showed better synovialization, with the difference being statistically significant. For functional assessment of the knee joint, preand postoperative (at the final follow-up) Lysholm, Tegner activity, and Lachman scores were compared between groups. Although the scores were considerably improved from the preoperative values, the patients' clinical functional scores and stability scores were not statistically significantly correlated with the area of synovial coverage.
The main limitations of this study include the small number of cases and relatively short follow-up period. Furthermore, the method of measuring the area of synovial coverage was subjective.
In conclusion, the age of the patient, length of preserved remnant tissue of the torn ACL, type of bundle, type of graft, and time from injury to surgery were found to influence synovialization of the grafted tendon after double-bundle ACL reconstruction. However, functional scores, such as Lysholm and Tegner activity scores, were not associated with synovialization.
Figures and Tables
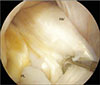 | Fig. 1Representative second-look arthroscopic image showing synovialization at 1 year after double-bundle reconstruction of a torn anterior cruciate ligament. Compared to the posterolateral (PL) bundle, the anteromedial (AM) bundle appears enveloped with abundant synovium. |
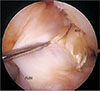 | Fig. 2Graft tendon synovialization at 1 year after double-bundle reconstruction of a torn anterior cruciate ligament. Compared to the allograft (Allo), the autograft (Auto) appears thicker and curved with more abundant capillary infiltration by the synovium. |
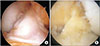 | Fig. 3Second-look arthroscopic images showing synovialization of tendons treated at different times after injury. Reconstruction in the acute stage (A) provides superior outcomes compared to those obtained after reconstruction in the chronic stage (B). |
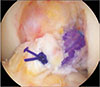 | Fig. 4Suturing along the remnant tissue of the anterior cruciate tendon at the tibial side. The remnant tendon was sutured with absorbable 2-0 polydioxanone, and the sutures cover the grafted ligament. |
 | Fig. 5Histology of synovium samples from grafted tendons at 1 year after double-bundle reconstruction of a torn anterior cruciate ligament. Synovial lining cells (arrows) are much more abundant in the autograft specimen (A) than in the allograft specimen (B) (H&E, magnification ×40). |
Table 1
Synovialization of the Grafted Tendon on Second-Look Arthroscopy after Double-Bundle Anterior Cruciate Ligament Reconstruction

References
1. Yasuda K, Tanabe Y, Kondo E, Kitamura N, Tohyama H. Anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2010; 26:9 Suppl. S21–S34.

2. Ochi M, Abouheif MM, Kongcharoensombat W, Nakamae A, Adachi N, Deie M. Double bundle arthroscopic anterior cruciate ligament reconstruction with remnant preserving technique using a hamstring autograft. Sports Med Arthrosc Rehabil Ther Technol. 2011; 3:30.

3. Kondo E, Yasuda K, Onodera J, Kawaguchi Y, Kitamura N. Effects of remnant tissue preservation on clinical and arthroscopic results after anatomic double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2015; 43(8):1882–1892.

4. Ahn JH, Choi SH, Wang JH, Yoo JC, Yim HS, Chang MJ. Outcomes and second-look arthroscopic evaluation after double-bundle anterior cruciate ligament reconstruction with use of a single tibial tunnel. J Bone Joint Surg Am. 2011; 93(20):1865–1872.

5. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017; 25(4):1205–1210.

6. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016; 44(7):1708–1716.

7. Tajima T, Chosa E, Yamaguchi N, Taniguchi N, Ishida Y. Remnant-preserving, selective single-bundle augmentation of the anterior cruciate ligament using a bone-patellar tendon-bone autograft: a technical note. Knee. 2016; 23(3):554–558.

8. Lee BI, Kwon SW, Choi HS, Chun DI, Kim YB, Kim BM. Anatomic single-bundle anterior cruciate ligament reconstruction with remnant preservation using outside-in technique. Arthrosc Tech. 2015; 4(4):e331–e334.

9. Schultz RA, Miller DC, Kerr CS, Micheli L. Mechanoreceptors in human cruciate ligaments: a histological study. J Bone Joint Surg Am. 1984; 66(7):1072–1076.

10. Ochi M, Iwasa J, Uchio Y, Adachi N, Sumen Y. The regeneration of sensory neurones in the reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 1999; 81(5):902–906.

11. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair: defining a rationale for augmentation. Am J Sports Med. 1991; 19(3):243–255.

12. Schutte MJ, Dabezies EJ, Zimny ML, Happel LT. Neural anatomy of the human anterior cruciate ligament. J Bone Joint Surg Am. 1987; 69(2):243–247.

13. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008; 24(5):560–568.

14. Ochi M, Adachi N, Deie M, Kanaya A. Anterior cruciate ligament augmentation procedure with a 1-incision technique: anteromedial bundle or posterolateral bundle reconstruction. Arthroscopy. 2006; 22(4):463.e1–463.e5.

15. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005; 21(1):19–24.

16. Fremerey RW, Lobenhoffer P, Zeichen J, Skutek M, Bosch U, Tscherne H. Proprioception after rehabilitation and reconstruction in knees with deficiency of the anterior cruciate ligament: a prospective, longitudinal study. J Bone Joint Surg Br. 2000; 82(6):801–806.
17. Ahn GY, Nam IH, Moon GH, Lee YH, Choi SP, Yoo JY. The effect of a tibial remnant preservation technique on the synovialization of the graft tendon in anterior cruciate ligament reconstruction: based on the second look arthroscopic findings. J Korean Arthrosc Soc. 2013; 17(1):11–17.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download