INTRODUCTION
METHODS
Intestinal epithelial cell culture and LPS-treated stress
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Measurement of reactive oxygen species (ROS) formation
Western blot assay
Animals
Induction of colitis and treatment
Statistical analysis
RESULTS
Exogenous IGFBP-3 expression in the mouse colon by the intracolonic administration of adenoviral particles
 | Fig. 1The organ staining for LacZ expression and the tissue immunohistochemistry staining for IGFBP-3 expression. (A) Rectal administration is the most effective method to express of LacZ in the colon (up; liver, down; colon). (a) Control, (b) intraperitoneal administration, (c) intravenous administration, (d) rectal administration. (B) The immunohistochemistry stain for detecting IGFBP-3 expression shows rectal administration is the most effective method. (a) Control, (b) intraperitoneal administration, (c) intravenous administration, (d) rectal administration (× 200).
IGFBP-3 = insulin-like growth factor-binding protein-3.
|
Increased IGFBP-3 expression inhibits LPS-induced cell cytotoxicity in IEC-6 cells
 | Fig. 2MTT assay and ROS formation in IEC-6 cells. (A) Cell viability was measured using the MTT assay after 24 hours of LPS stress (10 μg/mL) in IEC-6. Cell viability = A treatment/A control. The values were reported as statistical mean. Control, 1.0000; Ad/LacZ, 0.9976; Ad/IGFBP-3, 0.9965; LPS, 0.9288; LPS + Ad/LacZ, 0.9344; LPS + Ad/IGFBP-3, 0.9484. LPS + Ad/IGFBP-3 group shows higher viability than other two injury groups (LPS and LPS + Ad/LacZ). The values were reported as the mean ± SE. The LPS + Ad/IGFBP-3 group was compared to LPS + Ad/LacZ group. P < 0.05 was calculated using unpaired Student's t-test. (B) Flow cytometric histogram shows the high levels of ROS formation in response to LPS stress. The Ad/IGFBP-3 therapy reduced the ROS formation by a left-shift. The representative data were presented from ten independent experiments were performed.
MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, ROS = reactive oxygen species, IEC-6 = intestinal epithelial cell 6, LPS = lipopolysaccharides, Ad/IGFBP-3 = adenoviral vector system expressing insulin-like growth factor-binding protein-3, SE = standard error.
|
IGFBP-3 expression lowers the level LPS-stimulated IEC-6
IGFBP-3 suppresses the expression of inflammatory cytokines
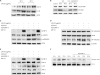 | Fig. 3The Western blots in IEC-6 cells. (A) COX-2 and IL-1β levels in total cell lysate was determined using Western blot analysis. The cells were treated with LPS (0–10 μg/mL) for 24 and 48 hours. The expression of both COX-2 and IL-1β are increased according to cell damage or exposure duration. (B) The protein levels of Cu/Zn SOD and Mn SOD as an antioxidant enzyme were examined. The expressions were checked at 24 and 48 hours after LPS stress (10 μg/mL). Pretreated Ad/IGFBP-3 group maintained the levels of SOD enzyme. (C) Phospho-ERK and Phospho-p38MAPK signaling were measured. The adenoviral vector Ad/IGFBP-3 reduced the expression of P-p38MAPK and P-ERK. (D) The NF-κB activity was measured. The adenoviral vector Ad/IGFBP-3 reduced translocation of NF-κB from the cytosol to the nucleus. (E) Total cell lysate was measured using Western blot analysis. Pretreatment with Ad/IGFBP-3 reduced the expression of these pro-inflammatory cytokines (COX-2, IL-1β, TNF-α). (F) Treated with Ad/IGFBP-3 reduced the expression of phospho-IκB-α.
IEC-6 = intestinal epithelial cell 6, SOD = superoxide dismutase, LPS = lipopolysaccharides, IL = interleukin, COX = cyclooxygenase, Ad/IGFBP-3 = adenoviral vector system expressing insulin-like growth factor-binding protein-3, NF-κB = nuclear factor-κB, TNF-α = tumor necrosis factor-α.
|
Ad/IGFBP-3 treatment ameliorates the severity of DSS-induced colitis
 | Fig. 4The change of body weights. On day 3 and 6, 1 × 1011 pfu (200 μL) Ad/LacZ or Ad/IGFBP-3 was administered per rectal. Body weights were examined on the same time of the day. The DSS + Ad/IGFBP-3 group compared to the DSS + Ad/LacZ group. Pretreated Ad/IGFBP-3 group shows higher body weights than Ad/LacZ treated group's one on day 10 (P = 0.028). Mean ± SE. P < 0.05 was calculated by unpaired Student's t-test.
Ad/IGFBP-3 = adenoviral vector system expressing insulin-like growth factor-binding protein-3, DSS = dextran sodium sulfate, SE = standard error.
|
 | Fig. 5The change of colonic length. Colons were harvested on day 10, and colonic lengths were measured from the cecum to the rectum. The Ad/IGFBP-3-treated group compared to the DSS group or Ad/LacZ-treated group. Pretreated Ad/IGFBP-3 group shows higher colonic length than the other two group's one significantly (aP = 0.016, bP = 0.032). Statistical mean. aP < 0.05 and bP < 0.05 were calculated by unpaired Student's t-test.
Ad/IGFBP-3 = adenoviral vector system expressing insulin-like growth factor-binding protein-3, DSS = dextran sodium sulfate.
|
Ad/IGFBP-3 treatment provides a protective effect in the colon of DSS-treated mice
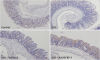 | Fig. 6Immunohistochemical staining for IGFBP-3 (× 200). IGFBP-3 expression. Pretreated Ad/IGFBP-3 induced the expression of IGFBP-3 in colonic mucosal cells.
IGFBP-3 = insulin-like growth factor-binding protein-3, Ad/IGFBP-3 = adenoviral vector system expressing insulin-like growth factor-binding protein-3, DSS = dextran sodium sulfate.
|
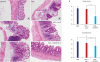 | Fig. 7The histologic findings and score of colonic mucosa. (A-D) Ad/IGFBP-3 treatment prevented DSS-induced damage in histologic evaluation. (A) Control, (B) DSS, (C) DSS + Ad/LacZ, (D) DSS + Ad/IGFBP-3. (D) DSS + Ad/IGFBP-3 group shows sustained crypt structure compared to the other DSS injury groups (hematoxylin-eosin stain, × 200). (E) Histological scoring of the colitis was performed. The severity was quantified by two scoring systems. The crypt abnormality and the Involvement of inflammation were significantly reduced in the Ad/IGFBP-3-treated group compared with Ad/LacZ-treated group. Statistical mean of crypt score and involvement score. DSS (3.67 and 3.67), DSS with Ad/LacZ (3.50 and 3.33), DSS with Ad/IGFBP-3 (1.83 and 2.67). aP = 0.009, bP = 0.014, and cP = 0.056 were calculated by Mann-Whitney U test.
Ad/IGFBP-3 = adenoviral vector system expressing insulin-like growth factor-binding protein-3, DSS = dextran sodium sulfate.
|




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download