INTRODUCTION
METHODS
Study subjects and protocol
 | Fig. 1Steps of the study protocol: 1) All participants in the KLAP and/or KEQAS were categorized into three laboratory subgroups based on their standardization level, such as HSL, BSL, and NSL; 2) New diagnostic cutoff values for DM and IFG which could provide FP and FN were calculated; and 3) The prevalence of DM and IFG were estimated using the new diagnostic cutoff values, and compared among laboratory subgroups.
DM = diabetes mellitus, IFG = impaired fasting glucose, KNHANES = Korea National Health and Nutrition Examination Survey, KLAP = Korean Laboratory Accreditation Program, KEQAS = Korean External Quality Assessment Scheme, HSL = highly-standardized laboratory, BSL = basically-standardized laboratory, NSL = non-standardized laboratory, ADA = American Diabetes Association, VIS = variance index score, FP = false positive, FN = false negative.
|
Diagnostic glucose cutoff values for DM and IFG
 | Fig. 2Relationship between the change in diagnostic cutoff values for diabetes mellitus and its induced measurement error, regarding FP and FN.
FP = false positive, FN = false negative, VIS = variance index score, CCV = chosen coefficient of variation.
|
Estimation of the prevalence of DM and IFG using data from KNHANES
Statistical analyses
RESULTS
Laboratory categorization by standardization level
Table 1
VIS of glucose according to laboratory standardization levels

| VIS of blood glucose | HSLb (na = 261) | BSLc (na = 981) | P value |
|---|---|---|---|
| Mean ± SD | 26.7 ± 18.1 | 43.7 ± 34.7 | < 0.001 |
| Median (IQR) | 21.2 (15.3–31.8) | 33.3 (21.9–52.2) | - |
Diagnostic glucose cutoff values for DM and IFG by laboratory subgroup
Table 2
Assumptions based on the virtual scenario for the diagnosis of DM and IFG

Estimated prevalence of DM and IFG using data from KNHANES
Table 3
Demographic characteristics of the subjects from KNHANES for Korean individuals over the age of 30

Table 4
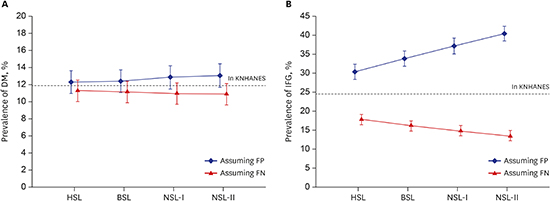
Estimated prevalence of DM and IFG for Korean individuals over the age of 30

 | Fig. 3Estimated prevalence of DM and IFG according to the standardized level assuming false positivity and false negativity. Each point and its vertical bar represents average prevalence and its 95% confidence interval. The dashed line indicates the prevalence in the KNHANES dataset, which is considered the reference value. Estimated prevalence of (A) DM and (B) IFG according to laboratory standardization level.
DM = diabetes mellitus, IFG = impaired fasting glucose, KNHANES = Korea National Health and Nutrition Examination Survey, FP = false positive, FN = false negative, HSL = highly-standardized laboratory, BSL = basically-standardized laboratory, NSL = non-standardized laboratory.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download