TA is one of the leading causes of pediatric RVH in Asia, however, 26%–70% of patients were asymptomatic and had hypertension found only incidentally.
1 Brunner et al.
2 demonstrated that the incidence of hypertension and elevated ESR were higher in 241 pediatric patients (82.6% and 61.0%) than in 844 adult patients (52.5% and 45.2%) with TA. Fieldston et al.
3 proposed that extensive imaging of the vascular system should be performed to achieve an early diagnosis of TA in children who presented with hypertension, elevated ESR, and systemic complaints. Even though, early diagnosis of c-TA remains a daunting challenge to pediatricians. Lately, the classification criteria for c-TA has been validated, including a mandatory criterion of angiographic abnormality plus at least one of the following five criteria: pulse deficit or claudication, blood pressure discrepancy in four limbs, bruits, hypertension, and elevated acute phase reactant.
4 Our patient's clinical presentations met the classification criteria of c-TA, including angiographic abnormality, systemic hypertension, and elevated acute phase reactant.
Treatments with corticosteroids, immunosuppressants, and biologics have been reported with success, however, there was no evidence showing the superiority of a single agent over another for the treatment of c-TA.
56 Disease progression raises questions about the necessity of revising current therapeutic strategies for c-TA, despite that immunosuppression could be achieved by corticosteroids, immunosuppressants, and biologics.
56 Corticosteroid can ameliorate active inflammation of c-TA, but it cannot cure RVH caused by RAS (RVH/RAS). Antihypertensive agents were only palliative for RVH/RAS. Because of the progressive nature of RAS, repeated PTRA has been advocated as a treatment of choice.
7 SI should be reserved as a bailout procedure for childhood RAS, if there was suboptimal results or arterial dissection after PTRA.
8
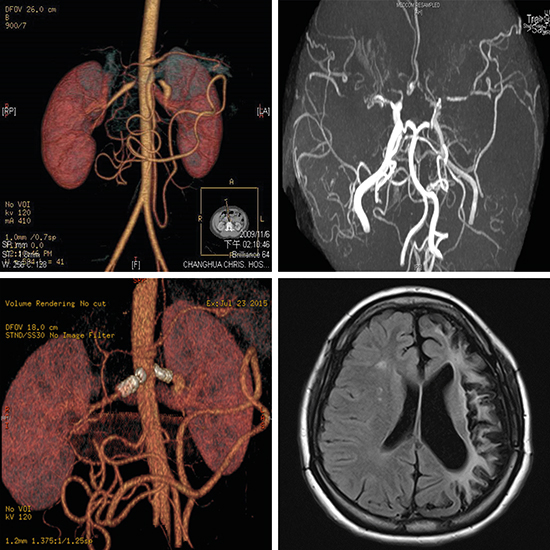
Moyamoya disease (MMD) is referred to an idiopathic occlusion of bilateral arteries of the circle of Willis, and MMS is defined as an underlying disease having a similar angiographic feature of a puff-of-smoke (moyamoya).
11 The incidence of MMS in c-TA is extremely low, and was reported only anecdotally in c-TA.
1112 There are four types of presentation in MMD (ischemic, hemorrhagic, epileptic, and other), and the ischemic type is predominant in children,
13 disregarding its underlying diseases. Surgical revascularization is the mainstay treatment for c-MMD, including direct procedure of STA-MCA anastomosis, indirect procedures of EMS, EDMS, and encephaloduroarteriosynangiosis, and a combination of both.
13 However, surgical revascularization could be a double-edge sword, since it can improve cerebral circulation and reduce the risk of subsequent cerebrovascular stroke and transient ischemic attack, but it may also cause postoperative neurological deterioration due to cerebral watershed shift or cerebral hyperperfusion syndrome (CHS).
13 CHS was defined as the presence of a significant increase in CBF at the site of the STA-MCA anastomosis which is responsible for the apparent neurologic sign.
14 The incidence of CHS, after anastomosis, was 5.3% in c-MMD
15 and 22.7% in adult-onset MMD,
16 disregarding its underlying diseases. The risk factors for CHS were left hemisphere operation, higher postoperative CBF, and higher postoperative/preoperative CBF ratio adjusted by cerebellar CBF.
16 Since CHS usually occurred 2–6 days after surgery, quantitative analysis of CBF by means of single-photon emission computed tomography, micro-Doppler ultrasonography, perfusion-weighted MRI, and time-of-flight MRA is highly recommended within 48 hours after surgery.
16 What orchestrates the pathogenesis of the CHS after STA-MCA anastomosis remains uncertain. The hypothesis may be increased pro-inflammatory cytokines, molecules, and intrinsic vulnerable microvascular structure (medial thinness and abnormality of the internal elastic lamina), excessive accumulation of pro-inflammatory cytokines and molecules (vascular endothelial growth factor, matrix metalloproteinase 9, etc.) and reactive oxygen products, and finally increased permeability of the blood-brain barrier.
17 Prophylactic blood pressure lowering (110–130 mmHg)
17 and minocycline (200 mg/day, a neuroprotective antibiotic that may ameliorate cerebral edema/hemorrhage by blocking matrix metalloproteinase 9),
18 can prevent focal neurological deterioration due to CHS. In the present case, the patient's blood pressure dropped to 128/86 mmHg after the first attempt of endovascular revascularization and was once kept in the recommended ranges of 110–130 mmHg.
17 However, decreasing blood pressure by endovascular revascularization, together with the intrinsic nature of ongoing active inflammation of c-TA,
19 may potentially render flare-ups of renal and cerebral artery stenosis in this patient. STA-MCA anastomosis provides favorable long-term outcomes,
15 however, an up-to-date one-stage surgery, including unilateral STA-MCA anastomosis, with EDMS, and bifrontal encephalo-duro-periosteal-synangiosis, has been proposed as an alternative to prevent neurocognitive decline in c-MMD.
20
In conclusion, the intrinsic nature of ongoing active inflammation of c-TA may render flare-ups of renal and cerebral artery stenosis. Decrease of blood pressure by endovascular revascularization and improvement of cerebral perfusion by surgical revascularization may have exposed the cerebral deep watershed zone to ischemia and/or CHS by a steal phenomenon (or Venturi effect), and caused focal neurological deficits in our patient. Revascularization could be a double-edge sword for concomitant cerebral and renal artery stenosis in c-MMS associated with c-TA, and should be performed with caution.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download