INTRODUCTION
Several oncogenic mutations in non-small cell lung cancer (NSCLC) have been discovered, directly impacting patient's prognosis and management. With the exception of well-known alterations, such as epidermal growth factor receptor (
EGFR) mutation or anaplastic lymphoma kinase (
ALK) rearrangement, genetic profiling has identified additional driver mutations in lung adenocarcinomas with 9%–14% being new targetable oncogenes.
1
Human epidermal growth factor receptor 2 (
HER2) is a member of the
ERBB receptor tyrosine kinase family.
HER2 receptor is activated by homodimerization or heterodimerization with the other
ERBB family receptors, especially
EGFR,
1 resulting in augmentation of
EGFR signaling. Oncogenic activation of
HER2 is present in various malignancies, such as breast, ovarian, bladder, salivary gland, endometrial, pancreatic, and NSCLC.
2 The major mechanisms of
HER2 alterations in NSCLC include
HER2 protein overexpression, gene amplification and mutation.
3 HER2 mutation is predominantly observed in female patients, non-smokers and patients with adenocarcinoma subtype, similar to
EGFR-mutated NSCLC.
4
The benefit of
HER2-targeted therapy for NSCLC harboring
HER2 alteration is much less defined than that for breast cancer. Trastuzumab, a monoclonal antibody that targets extracellular domain IV of
HER2 receptor, has not shown a clear benefit in
HER2-positive NSCLC.
5 By comparison, tyrosine kinase inhibitors (TKIs) that target both
EGFR and
HER2 have been associated with response to treatment.
5 Among them, afatinib (Gilotrif
®, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA) is a potent and irreversible
ERBB family blocker with preclinical activity in
Ba/F3 cells expressing an artificial
HER2 mutant and in a human lung cancer cell line with an insertional mutation at codon 776.
6 Results with afatinib in
HER2-positive NSCLC have been promising, but there have been only a few clinical studies and limited data in the form of case reports involving western patients.
7891011
We present the first oriental case of previously treated NSCLC, which initially had no available activating target and finally had response to afatinib after detection of HER2 mutation. Furthermore, we review the clinical impacts of HER2 alterations in diagnosis and the role of afatinib in treatment of NSCLC harboring HER2 mutation.
CASE DESCRIPTION
A 50-year-old Korean woman who had never smoked visited our lung cancer clinic in February 2015. She complained of upper airway infection symptoms of mild back pain followed by cough with sputum and rhinorrhea. Computed tomography (CT) from an outside hospital showed a right upper lobar mass with enlargement of adjacent interlobar lymph node, and miliary distribution of multiple tiny nodules in both lungs (
Fig. 1A). She was diagnosed with stage IV lung adenocarcinoma with metastases to both lungs, T12 spine, left femur and brain that was proven by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), positron emission tomography and brain magnetic resonance imaging (MRI). Her initial tumor biopsy from primary mass and lymph nodes was negative for
EGFR mutation and
ALK translocation.
 | Fig. 1
Serial CT scans of a present patient before treatment with afatinib. (A) Baseline image before chemotherapy. (B) Post-treatment image after four cycles of pemetrexed and cisplatin, assessed as stable disease. (C) Follow-up image without subsequent treatment before start of gefitinib, showing disease progression of right upper lobe mass and lung-to-lung metastases. (D) Post-treatment image after two cycles of gefitinib, assessed as progression with aggravation of lung-to-lung metastases.
CT = computed tomography.

|
She received four cycles of pemetrexed and cisplatin, and her tumor burden was retained as stable disease (
Fig. 1B). She stopped the chemotherapy due to poor performance status without maintenance therapy. Upon progression of right upper lobar mass, multiple lung nodules and metastatic lymphadenopathies after 2 months without subsequent treatment (
Fig. 1C), she received gefitinib for the next 2 months. Subjectively-rated symptoms of nausea and myalgia seemed to ease, but gefitinib was finally stopped due to aggravation of gastrointestinal toxicity and lung-to-lung metastases (
Fig. 1D). Thereafter, progression in brain metastasis was also demonstrated in follow-up MRI. She suffered from general weakness, nausea and back pain, and so was reluctant to receive further cytotoxic chemotherapy.
In September 2015, she agreed to be tested for another activating mutation using previously achieved specimen by EBUS-TBNA. Pathologic examination revealed a poorly differentiated atypical cell nest with focal glandular differentiation (
Fig. 2B). Immunohistochemical stain for
c-erbB2 showed weak membranous staining (
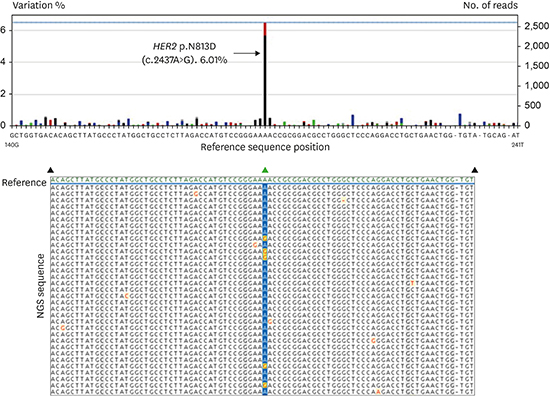
Fig. 2C). After direct sequencing as screening test, the following next generation sequencing (NGS) of the tissue revealed a
HER2 exon 20 mutation with A-to-G base change at nucleotide 2437 (c.2437A>G), which led to substitution of aspartate for asparagine at position 813 (p.N813D) (
Fig. 2A). This mutation has never been reported. In October 2015, she started afatinib 40 mg daily. An initial clinical and radiologic response was achieved within a month. She professed a sense of well-being and relief of back pain, and simple chest X-ray showed more decreased lung nodules grossly. During week 6 of treatment, a CT assessment revealed a partial response in extracranial lesions with prominent regression of primary lung tumors in the right upper lobe and lung-to-lung metastases (
Fig. 3). Brain metastases had maintained the stable state, and she did not complain of any neurologic symptom or sign. After stepwise dose reduction to 20 mg due to stomatitis and nausea, she has continued the medication until February 2016 without deterioration of subjective symptom and tumor burden. Last CT scan on January 2016 still showed a partial response in extracranial lesion, but brain MRI revealed mild increase of metastases. At last visit on February 2016, the patient complained of general weakness and anorexia with stomatitis, and thereafter she was lost to follow-up.
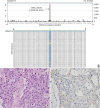 | Fig. 2
Pathologic and molecular diagnosis for lung adenocarcinoma harboring HER2 mutation. (A) Next-generation sequencing result of a patient with HER2 exon 20 mutation. The A-to-G base-pair change at nucleotide 2437 in HER2 exon 20 (c.2437A>G) is denoted by the dominant black bar which is found in approximately 6% of sequence reads. The sequence depth is 2614 indicated by the blue line. The A-to-G base change leads to substitution of aspartate for asparagine at position 813 (p.N813D). Variation less than 1% is regarded as experimental noise. Nucleotides are colored as follows: G as black bar, A as green bar, C as blue bar, T as red bar, and base deletion as gray bar. A selection of aligned sequence reads is shown with A-to-G base change highlighted in yellow with red lettering. First line shows the reference sequence (green). (B) Poorly differentiated atypical cell nest with focal glandular differentiation (H & E, × 400). (C) Weak membranous staining on immunohistochemical staining for c-erbB2 (× 400).
HER2 = human epidermal growth factor receptor 2, H & E = haematoxylin and eosin, NGS = next generation sequencing.

|
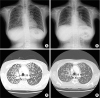 | Fig. 3
Radiologic findings before and after treatment. (A) Chest X-ray before starting afatinib. (B) X-ray 6 weeks after afatinib. (C) CT scan before starting afatinib. (D) CT scan 6 weeks after afatinib. The images demonstrate regression of right upper lung mass and lung-to-lung metastases.
CT = computed tomography.

|
DISCUSSION
We provide evidence of clinical benefit from treatment with afatinib in an oriental patient with HER2-mutant NSCLC who had previously failed cytotoxic chemotherapy and treatment with reversible EGFR inhibitor.
In terms of
HER2 gene mutation, NGS is becoming an important method of detection in NSCLC, even though the traditional single-gene sequencing (i.e., Sanger sequencing) is still widely performed. NGS can simultaneously test for multiple oncogenic driver mutations, rearrangements and gene amplifications.
5 In this case, we performed direct sequencing for screening followed by NGS for confirmation. And we found a
HER2 exon 20 mutation (p.N813D) which has never been reported. Although it takes more than three weeks to confirm the result and the cost is quite high in real clinic settings, NGS has advantage over direct sequencing in detecting very rare mutations even when the biopsy specimen is limited.
There were some reports about types of
HER2 mutation. Compared with
EGFR,
HER2 mutations were less heterogeneous. The majority (over 80%) were in-frame insertions in exon 20 of the kinase domain, which ranged from 3 to 12 base-pair (bp), all nested between codons 775 and 831.
12 The 12-bp insertion is known as the most common mutation showing duplication/insertion of 4 amino acids (YVMA) at codon 775 (A775_G776insYVMA).
12 In addition, other mutations are as follows: bp insertion (i.e., G776>VC, P780_Y781insGSP, V777_G778insCG), insertion/deletion (i.e., M774delinsWLV, G776>LC), or point mutations (i.e., L755S, G776C, G776L, V777L).
791213 There was no previous report about N813D point mutation found in this case. N813D mutation is also located in exon 20 of
HER2 kinase domain. Given that most
HER2 mutations were known to be found in the proximal region of exon 20, N813D is supposed to be a novel and rare mutation in the distal region of exon 20.
Available data concerning
HER2-targeted therapy for
HER2-positive NSCLC is insufficient and less defined. Trastuzumab monotherapy or addition to chemotherapy has not shown a clear benefit in
HER2-positive NSCLC,
5 although certain cases with favorable outcome have been described.
914 Several dual TKIs to
EGFR and
HER2 have shown hopeful results in select patients. In a recent phase II cohort study for recurrent or newly detected lung adenocarcinoma, dacomitinib produced objective responses in patients with specific
HER2 exon 20 insertion mutation (response rate 12%, median overall survival 9 months).
13 Neratinib in combination with temsirolimus also showed responses in two of 11 patients with
HER2 mutation-positive NSCLC in a phase I study of pretreated patients with
HER2-driven cancers.
15
The National Comprehensive Cancer Network has included trastuzumab and afatinib as potential therapy options for NSCLC patients with
HER2 mutations.
16 Afatinib, a pan-
HER inhibitor, has shown limited but promising data for treatment of
HER2-mutated NSCLC from several reports and studies mostly involving western patients, including Europe, United States, and Australia. In an exploratory phase II study with afatinib, all three patients with
HER2 mutation showed objective response, even after failure of other
EGFR- and/or
HER2-targeted treatment.
7 In a recent case report, a patient harboring specific
HER2 mutated lung adenocarcinoma with exon 20 YVMA insertion (p.A775_G776insYVMA) showed durable response for 10 months to afatinib; the patient had been alternately treated with cytotoxic chemotherapy and
HER2-targeted agents including pertuzumab and trastuzumab.
8 A retrospective series of 16 patients with
HER2 mutation showed favorable outcomes, with a 93% disease control rate for trastuzumab-based therapies and 100% for afatinib.
9 In a recent phase II study of afatinib for previously treated lung adenocarcinoma, five of seven patients achieved disease control (71%). Furthermore, one patient who received afatinib (40 mg/day) combined with paclitaxel following afatinib monotherapy (50 mg/day) due to progression showed a confirmed partial response with a duration of 41.9 weeks.
11 These data demonstrate that afatinib may have clinical application in patients whose tumors are constitutively dependent on the
HER2 pathway. Similarly, in the present report, which is the first case in an eastern population, the patient showed a rapid regression within a month and durable response over 5 months. Although dose reduction was inevitable due to adverse events beginning a month after commencement of treatment, the objective response was continued thereafter even when the initial dose of afatinib was halved (20 mg/day).
The antitumor mechanism of afatinib in
HER2-altered NSCLC has been demonstrated by in vitro and in vivo experimental study. In a study using
HER2-altered NSCLC cell lines and xenograft mouse model, afatinib downregulated the phosphorylation of
HER2 and
EGFR in addition to their downstream signaling, and induced and antiproliferative effect through G1 arrest and apoptotic cell death both in
HER2-amplified and -mutant cell lines, which was also confirmed in vivo.
17
However, the superiority of a
HER2-targeted therapy to chemotherapy is not conclusive. In a European cohort study for advanced NSCLC with
HER2 exon 20 insertion mutation, subgroup with conventional therapy including chemotherapy and reversible
EGFR-TKI did not show a survival benefit compared to those with
HER2-targeted drugs including trastuzumab (monotherapy or addition to chemotherapy), trastuzumab emtasine (T-DM1), neratinib, afatinib and lapatinib.
10 Therefore, additional well-designed randomized clinical trials with novel and target-specific agents are warranted.
In summary, afatinib could be a potential treatment option for subgroups of HER2-mutated NSCLC that progress after previous cytotoxic chemotherapy or treatment with reversible EGFR-TKIs. NGS could be advantageous as it provides a precise result for HER2 mutation, even in limited biopsy specimen.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download