INTRODUCTION
CASE DESCRIPTION
KPD matching rules in Samsung Medical Center
Table 1
The characteristics of KPD recipients and donors
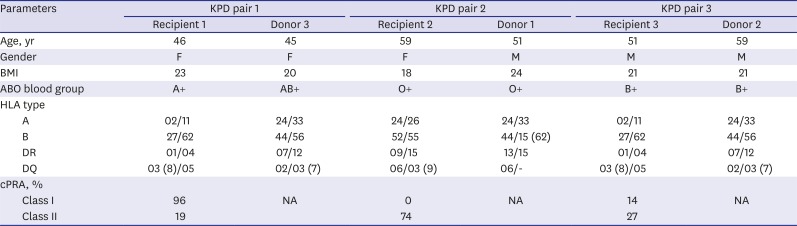
Table 2
Matching conditions of KPD transplantation for original and KPD pairs
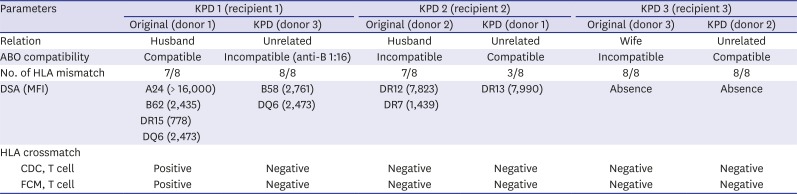
Table 3
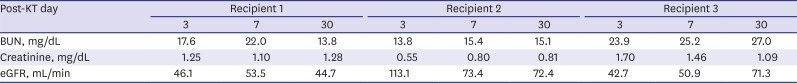
The outcome of KPD
Journal List > J Korean Med Sci > v.33(5) > 1107703



Author Contributions: Conceptualization: Kang ES. Data curation: Yu S, Chun K, Cho CW, Lee N, Lee KW. Investigation: Huh W, Jang HR. Writing - original draft: Oh D. Writing - review & editing: Park H, Park JB, Kim SJ.
Dongkyu Oh 
https://orcid.org/0000-0002-1476-2554
Eun-Suk Kang 
https://orcid.org/0000-0001-6386-6520
Shinae Yu 
https://orcid.org/0000-0002-9527-5853
Kyoungsuk Chun 
https://orcid.org/0000-0003-3261-9863
Wooseong Huh 
https://orcid.org/0000-0001-8174-5028
Hye Ryoun Jang 
https://orcid.org/0000-0001-9856-6341
Chan Woo Cho 
https://orcid.org/0000-0002-3546-8442
Nuri Lee 
https://orcid.org/0000-0003-3027-1592
Kyo Won Lee 
https://orcid.org/0000-0002-2722-7817
Hyojun Park 
https://orcid.org/0000-0002-5291-1338
Jae Berm Park 
https://orcid.org/0000-0001-9117-2278
Sung Joo Kim 
https://orcid.org/0000-0001-9251-1673