INTRODUCTION
Caffeine citrate is one of the widely used drugs in neonatal intensive care units. It stimulates breathing before infants are weaned from mechanical ventilation and reduces the frequency of apnea in preterm infants.
1 Caffeine has been found to have favorable effects in preterm infants who have patent ductus arteriosus (PDA). For example, reduced incidence of extubation failure and bronchopulmonary dysplasia and reduced need for surgical treatment were observed, compared with preterm infants who did not have it.
1 In a recent meta-analysis of 5 cohort studies, early caffeine therapy within 3 days of life was associated with a 29% decrease in incidence of PDA and a 59% decrease in PDA that required surgical treatment for closure. Although no beneficial effects on PDA were observed in a meta-analysis of 2 randomized trials.
2 Another meta-analysis reports early caffeine therapy triggers better clinical outcomes in the infants born with very low birth weight, without increasing the risk for necrotizing enterocolitis.
3 Despite these favorable clinical outcomes for PDA resulting from caffeine therapy in preterm infants, several studies have reported the immediate influence of caffeine loading doses in significantly decreasing cerebral blood flow velocity and cerebral oxygenation.
45
The present investigation was designed to assess the short-term hemodynamic effects on systemic blood flow and ductal shunting flow according to patency of ductus arteriosus after preterm infants received a single loading dose of 20 mg/kg of intravenous caffeine citrate (the equivalent to 10 mg/kg of caffeine base).
METHODS
Study design
Patients were preterm infants who were born at less than 37 weeks' gestational age and admitted to a tertiary-level neonatal intensive care unit between January 1, 2015 and December 31, 2016. We identified 54 infants for whom caffeine had been administrated to treat apnea or to wean them from mechanical ventilation. We excluded infants who had severe intraventricular hemorrhage, congenital heart disease except patent foramen ovale (PFO), and other congenital anomalies were excluded. All caffeine was administered intravenously in a dose of 20 mg/kg for 30 minutes. The modes of mechanical ventilation were not altered during study to minimize confounding variables for cardiac output.
We divided participating infants into 2 groups based on the presence of PDA: 25 preterm infants had PDA, including 8 who had symptomatic PDA (sPDA). We confirmed the diagnosis of PDA by color-flow Doppler echocardiography. sPDA was defined as the presence of 2 of 5 clinical signs with the confirmation of large left to right ductal flow.
6 The clinical signs of sPDA includes following features: 1) the presence of a systolic or continuous murmur, 2) a bounding pulse or a hyperactive precordial pulse, 3) difficulty in maintaining the blood pressure (BP), i.e., hypotension (the lower limit of normal mean arterial pressure was regarded to corrected gestational age) without response to loading fluid and infusion of dopamine, 4) a worsening ventilator status, and 5) chest radiographic evidence, i.e., pulmonary congestion or cardiomegaly (a cardiothoracic ratio > 60%) with increased pulmonary flow. The study is a prospective observational study with a post-hoc analysis based on the presence of PDA.
Measuring the hemodynamic parameters
A single investigator performed all echocardiographic studies immediately before and at 1 hour and 4 hours after an intravenous caffeine infusion. There were no significant differences between the 2 measurements, which were performed 5 to 10 minutes apart by the identical observer, for all parameters (median value differences P > 0.05). Mean variability between the measurements was within 20% for all parameters and 90% of the measures were within approximately 15% of each other.
Echocardiographic evaluation included 2-dimensional, M-mode, color-flow, and color-flow Doppler to assess right ventricular output (RVO), left ventricular output (LVO), superior vena cava (SVC) flow, ejection fraction (EF), shortening fraction (SF), and the left atrial to aortic ratio (LA/Ao ratio), with average of measured value from 3 to 5 cardiac cycles.
We conducted aortic Doppler via apical long-axis view, measured diameter via parasternal long-axis view, and measured proximal and below the aortic valve annulus. We conducted SVC Doppler by subcostal sagittal views, measured diameter via the parasternal 3-vessel view, and measured close to the entrance to the right atrium.
To evaluate left ventricular systolic function, we measured SF and EF based on M-mode. In addition, to evaluate cardiac output, we used Doppler to estimate the LVO based on left ventricular stroke volume.
7
In the preterm infants with PDA, we also measured additional parameters of ductus arteriosus, including diameter, maximal velocity (Vmax), velocity time integral (VTI, area measured under the Doppler velocity envelope for single heart beat), ductal shunting volume per beat (DSV) to analyze the effects of caffeine on ductus arteriosus.
Calculated ductal shunting flow is defined as increasing DSV and, concurrently, the heart rate (HR). In the presence of PDA, SVC flow is used as a surrogate for systemic blood flow.
78
Statistical analysis
Clinical data are presented as mean (± standard deviation), frequency, and percentage. As noted, we divided participating infants into 2 groups, those with and without PDA, to identify any hemodynamic changes between the groups. We used repeated-measures analysis of variance (ANOVA) to analyze the hemodynamic parameters at baseline and after 1 hour and 4 hours. We also analyzed the significant contributors to ductus arteriosus using the paired t-test to compare changes. We conducted all t-test comparisons using the values before the caffeine was loaded. We defined statistical significance as P < 0.05 and used SPSS version 20 for the analyses (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was approved by the Institutional Review Board of Korea University Medical Center, Ansan Hospital by AS12031-002. Informed consent was provided by parents or guardians for the study.
RESULTS
We studied a total of 54 preterm infants, 25 of whom were boys. All infants were born before 37 gestational weeks, and 33 were born before 30 weeks; the preterm infants born before 30 weeks weighed lower than 1,500 g. The enrolled preterm infants were administered caffeine on 5.7 (± 5.4) day-of-life in the current study.
Patient characteristics
Table 1 demonstrates features of patients categorized by patency of ductus arteriosus. The preterm infants we studied had a mean (± standard deviation) gestational age of 29.6 weeks (± 13.3) and a mean birth weight of 1,404 g (± 379). Forty-nine infants had been exposed to antenatal steroids, and 42 (77.8%) had been delivered by caesarean section. The patients were administered caffeine 5.7 days (± 5.4) after birth. Twenty-five (46.3%) infants showed sustained PDA at time of the study, but only 8 (14.8%) had sPDA. Thirty-two (59.3%) infants had been intubated and were on positive pressure ventilation; 14 (25.9%) infants were receiving nasal continuous positive airway pressure and 8 (14.8%) required no respiratory support.
Table 1
Features of patients categorized by patency of ductus arteriosus (n = 54)

|
Characteristics |
Preterm infants with PDA (n = 25) |
Preterm infants without PDA (n = 29) |
|
Male/female |
12/13 |
12/17 |
|
Gestational age, wk |
29.2 ± 13.5 |
30.0 ± 12.7 |
|
Birth weight, g |
1,340 ± 364 |
1,459 ± 382 |
|
Vaginal delivery/caesarean section |
6/19 |
6/23 |
|
Antenatal steroids |
22 (88.0) |
27 (93.1) |
|
Twin |
8 (32.0) |
3 (10.3) |
|
Apgar score, 1 min |
5 [2–8] |
4 [0–9] |
|
Apgar score, 5 min |
7 [4–9] |
7 [2–10] |
|
Day of lifea
|
3.9 ± 1.7 |
7.3 ± 6.8 |
|
Need for respiratory supporta
|
23 (92.0)b
|
23 (79.3)c
|
The effects of caffeine in preterm infants with PDA
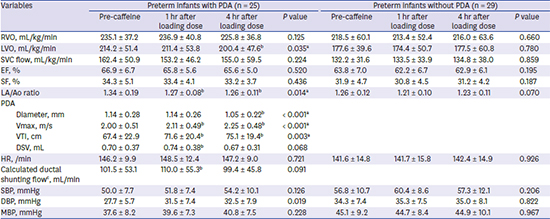
Table 2 summarizes the changes in hemodynamic parameters in preterm infants according to PDA.
Table 2
Changes in hemodynamic parameters based on PDA

|
Variables |
Preterm infants with PDA (n = 25) |
Preterm infants without PDA (n = 29) |
|
Pre-caffeine |
1 hr after loading dose |
4 hr after loading dose |
P value |
Pre-caffeine |
1 hr after loading dose |
4 hr after loading dose |
P value |
|
RVO, mL/kg/min |
235.1 ± 37.2 |
236.9 ± 40.8 |
225.8 ± 36.8 |
0.125 |
218.5 ± 60.1 |
213.4 ± 52.4 |
216.0 ± 63.6 |
0.660 |
|
LVO, mL/kg/min |
214.2 ± 51.4 |
211.4 ± 53.8 |
200.4 ± 47.6b
|
0.035a
|
177.6 ± 39.6 |
174.4 ± 50.7 |
177.5 ± 60.8 |
0.780 |
|
SVC flow, mL/kg/min |
162.4 ± 50.9 |
153.2 ± 46.2 |
155.0 ± 59.5 |
0.224 |
132.2 ± 31.6 |
133.5 ± 33.9 |
134.8 ± 38.0 |
0.859 |
|
EF, % |
66.9 ± 6.7 |
65.8 ± 5.6 |
65.6 ± 5.0 |
0.520 |
63.8 ± 7.0 |
62.2 ± 6.7 |
62.9 ± 6.1 |
0.195 |
|
SF, % |
34.3 ± 5.1 |
33.4 ± 4.1 |
33.2 ± 3.7 |
0.436 |
31.9 ± 4.7 |
30.8 ± 4.5 |
31.2 ± 4.2 |
0.187 |
|
LA/Ao ratio |
1.34 ± 0.19 |
1.27 ± 0.08b
|
1.26 ± 0.11b
|
0.014a
|
1.26 ± 0.12 |
1.21 ± 0.10 |
1.23 ± 0.11 |
0.070 |
|
PDA |
|
|
|
|
|
|
|
|
|
Diameter, mm |
1.14 ± 0.28 |
1.14 ± 0.26 |
1.05 ± 0.22b
|
< 0.001a
|
|
|
|
|
|
Vmax, m/s |
2.00 ± 0.51 |
2.11 ± 0.49b
|
2.25 ± 0.48b
|
< 0.001a
|
|
|
|
|
|
VTI, cm |
67.4 ± 22.9 |
71.6 ± 20.4b
|
75.1 ± 19.4b
|
0.003a
|
|
|
|
|
|
DSV, mL |
0.70 ± 0.37 |
0.74 ± 0.38b
|
0.67 ± 0.31 |
0.068 |
|
|
|
|
|
HR, /min |
146.2 ± 9.9 |
148.5 ± 12.4 |
147.2 ± 9.0 |
0.721 |
141.6 ± 14.8 |
141.7 ± 15.8 |
142.4 ± 14.9 |
0.926 |
|
Calculated ductal shunting flowc, mL/min |
101.5 ± 53.1 |
110.0 ± 55.3b
|
99.4 ± 45.8 |
0.091 |
|
|
|
|
|
SBP, mmHg |
50.0 ± 7.7 |
51.8 ± 7.4 |
54.2 ± 10.1 |
0.126 |
56.8 ± 10.7 |
60.4 ± 8.6 |
57.3 ± 12.1 |
0.206 |
|
DBP, mmHg |
27.7 ± 5.7 |
31.5 ± 7.4 |
32.5 ± 7.9 |
0.019 |
34.3 ± 7.4 |
35.3 ± 7.5 |
35.0 ± 8.1 |
0.822 |
|
MBP, mmHg |
37.6 ± 8.2 |
39.6 ± 7.3 |
40.8 ± 7.5 |
0.228 |
45.1 ± 9.2 |
44.7 ± 8.4 |
44.9 ± 10.1 |
0.967 |
Among the infants with PDA, we observed progressive decreases in LVO (P = 0.035) 4 hours after the caffeine loading. There was no difference the value before caffeine loading, at 1 hour and 4 hours. (P = 0.576 and P = 0.042 by paired t-test, respectively). SVC flow fell at 4 hours after caffeine loading without statistical significance (P = 0.224). In our study, SVC flow decreased at 1 hour but recovered at 4 hours compared with the values before caffeine loading (P = 0.094 and P = 0.237 by paired t-test, respectively).
We also observed decreased LA/Ao ratios (P = 0.014) 4 hours after caffeine loading. The ratios decreased significantly only at 4 hours and compared with the values before caffeine loading (P = 0.038 by paired t-test).
The diameters of the ductus arteriosus decreased in this study (P < 0.001), despite the significant increases in Vmax and VTI (P < 0.001 and P = 0.003, respectively). DSV through the ductus arteriosus significantly increased 1 hour (P = 0.027 by paired t-test) but recovered 4 hours after caffeine loading with ductal constriction (P = 0.538 by paired t-test). Calculated ductal shunting flow was increased and recovered, similar to changes of DSV regarding the effects of ductal constriction, with no significant changes in HR at 1 hour (P = 0.027 by paired t-test) or 4 hours (P = 0.538 by paired t-test) after caffeine loading. Meanwhile, in preterm infants without PDA, there were no statistically significant changes in any hemodynamic parameters.
DISCUSSION
This single-center study showed that a standard intravenous loading dose of caffeine increased ductal shunting flow and reduced SVC flow (as a surrogate of systemic blood flow) in enrolled preterm infants with PDA. After we administered caffeine, this hemodynamic alteration was recovered at four hours after the loading. One study on the cerebrovascular impact of caffeine loading subjected 40 preterm infants.
4 It showed that an intravenous dose of 10 mg/kg caffeine base led to noticeable reductions in cerebral blood flow velocity based on Doppler ultrasound and in cerebral oxygenation based on near-infrared spectroscopy 1 hour after caffeine loading. In that study as well, these hemodynamic changes recovered after 4 hours.
4 Another study concerning effects of caffeine on systemic blood flow revealed that oral loading of caffeine reduced cerebral and intestinal blood flow velocity by Doppler ultrasound 2 hours after caffeine loading.
5 Caffeine inhibits potent vasodilating function of adenosine and may result in vasoconstriction of cerebral blood vessels. Our finding of decreased systemic blood flow is similar to the findings from previous studies, supporting the effects of caffeine on decreasing cerebral and intestinal blood flow during caffeine loading.
In the current study, we focused on the presence of PDA to investigate the effects of caffeine on ductus arteriosus. We found that in preterm infants with PDA, caffeine increased flow through the ductus arteriosus before constricting it 4 hours after caffeine loading.
Generally, caffeine may suppress prostaglandin production, adenosine function, and constricting the ductus arteriosus.
9 In an animal study, otherwise, caffeine did not directly constrict the ductus arteriosus in vitro, which causes discrepancies between in vitro and in vivo studies because of the ductus's shear stress and effects of circulating substances on ductus contractility.
10 However, there have been few reports on the precise changes in echocardiographic parameters in preterm infants after loading caffeine. In an observational study with 31 subjects with very low birth weight, hemodynamic effects including increased LVO, HR, and BP were reported after caffeine had been administered 9.2 days after birth. In that study, the hemodynamic effects were evaluated 47 ± 20 minutes after the administration, and preterm infants with PDA were excluded to identify the precise effects of caffeine on LVO without interference by either a right-to-left or left-to-right ductal shunt.
11 In the current study, we also investigated preterm infants without PDA to identify hemodynamic changes after caffeine loading. In contrast to previous findings, we observed no significant hemodynamic effects including LVO, SVC flow, HR, and BP in these infants.
In our current study, we observed lower LA/Ao ratios (
P = 0.028) 4 hours after caffeine loading despite decreased ductal shunting flow. The LA/Ao ratio expresses increased volume load on the left atrium, which is associated with blood shunting through the duct arteriosus, bypasses the right heart, and directly enters the main pulmonary artery to increase pulmonary blood flow.
12 Traditionally, the LA/Ao ratio was an indirect tool for assessing the impact of left-to-right shunts in infants with hemodynamically significant PDA (hsPDA); specifically, a ratio greater than 1.4 is related to hsPDA.
13 However, the ratio is less accurate in the presence of PDA due to left-to-right shunting through PFO or impaired left ventricular function. The LA/Ao ratio might also underestimate the size of the left atrium, countering the effects of ductal shunt flow.
14 In addition, measuring the LA/Ao ratio on the parasternal long-axis view shows discrepancies in the form of minimal technical changes in angle or position.
15 In PDA, the SVC flow more accurately assesses systemic blood flow.
16 Infants with hsPDA have lower systemic blood flow to the abdominal aorta and lower blood flow velocity in the celiac, superior mesenteric, and renal arteries despite high LVO.
17
We assumed that caffeine accelerates the velocity of ductal shunting flow with a constant PDA diameter 1 hour after caffeine loading. DSV through the ductus arteriosus increased at 1 hour without changes in HR. SVC flow fell at 1 hour, and over an hour, these hemodynamic changes reduced systemic blood flow, triggering hemodynamic instability in preterm infants. The Vmax and VTI increased 4 hours after caffeine loading, and the ductus arteriosus tended to constrict; caffeine loading directly reduces the diameter of the ductus arteriosus. Four hours after caffeine loading, SVC flow recovered despite the decreased LVO. Our study has shown hemodynamic alterations in preterm infants shortly after caffeine loading.
Owing to the predictable hemodynamic instability of high loading doses of caffeine, other approaches to reducing hemodynamic instability should be considered. One study divided a loading dose of 12.5 mg/kg caffeine base twice, 4 hours apart over 15 minutes; however, this dose only reduced cerebral blood flow 1 hour after the second loading, and otherwise, there were no significant changes in intestinal blood flow after divided loading in this case-control study.
18 The results from participants' blood sampling showed that divided loading may trigger a delayed increase in blood concentration. To avoid adverse effects such as decreased cerebral or intestinal blood flow, we should be concerned about caffeine's blood concentrations, too.
In our sub-analysis of the 8 preterm infants with sPDA among the 25 preterm infants with PDA to identify caffeine's hemodynamic effects, these infants showed similar results to those for the preterm infants with PDA, such as decreased SVC flow at 1 hour after caffeine loading (data are not shown); the SVC flow recovered after 4 hours. We also found decreased PDA diameter and increased ductal shunting flow 1 hour after caffeine loading, but we could not find statistical significance because of the small number of eligible infants. Additional studies are needed to understand the hemodynamic changes after caffeine administration in preterm with hsPDA.
This was a pilot study to evaluate short-term systemic blood flow and ductal shunting flow during and after caffeine loading in preterm infants with PDA. A larger randomized controlled trial is needed to understand the direct effects of caffeine on cardiac and cerebral vessels and other systemic circulation during and after caffeine loading.
This study suggests that a standard intravenous loading dose of caffeine for 30 minutes could trigger hemodynamic instability in preterm infants with PDA. After caffeine loading, the infants experienced hemodynamic changes such as increased ductal shunting flow and decreased SVC flow (as a surrogate for systemic blood flow). The changes worsened the hemodynamics in cerebral and intestinal blood flow in preterm infants with PDA, and these hemodynamic changes required 4 hours for recovery, associated with consequent ductal constriction. Close monitoring of hemodynamic changes would be needed to observe the risk for pulmonary over-circulation or systemic hypo-perfusion due to transient increasing ductal shunting flow during caffeine loading in preterm infants with PDA.
Therefore, we suggest that a study be conducted to detect the hemodynamic differences between the conventional loading duration of 30 minutes and longer durations of loading. Additionally, a randomized controlled trial blinded setup with echocardiographic measurements in placebo group versus a caffeine group is needed to understand the hemodynamic impacts on cerebral perfusion and systemic oxygenation during and after caffeine loading, leading to better clinical prognoses in preterm infants who have hsPDA.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download