1. Dhawan V, Healy DG, Pal S, Chaudhuri KR. Sleep-related problems of Parkinson's disease. Age Ageing. 2006; 35(3):220–228.

2. Tandberg E, Larsen JP, Karlsen K. Excessive daytime sleepiness and sleep benefit in Parkinson's disease: a community-based study. Mov Disord. 1999; 14(6):922–927.

3. Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007; 22(11):1623–1629.

4. Askenasy JJ. Sleep disturbances in Parkinsonism. J Neural Transm (Vienna). 2003; 110(2):125–150.

5. Pal PK, Thennarasu K, Fleming J, Schulzer M, Brown T, Calne SM. Nocturnal sleep disturbances and daytime dysfunction in patients with Parkinson's disease and in their caregivers. Parkinsonism Relat Disord. 2004; 10(3):157–168.

6. Marinus J, Visser M, van Hilten JJ, Lammers GJ, Stiggelbout AM. Assessment of sleep and sleepiness in Parkinson disease. Sleep. 2003; 26(8):1049–1054.

7. Hughes AJ, Daniel SE, Lees AJ. The clinical features of Parkinson's disease in 100 histologically proven cases. Adv Neurol. 1993; 60:595–599.
8. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967; 17(5):427–442.

9. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003; 18(7):738–750.
10. Kang Y, Na DL, Hahn S. A validity study on the Korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15(2):300–308.
11. Lee JY, Cho SJ, Na DL, Kim SK, Youn JH, Kwon M, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008; 21(2):104–110.

12. Ahn YM, Lee KY, Yi JS, Kang MH, Kim DH, Kim JL, et al. A Validation study of the Korean -version of the montgomery- asberg depression rating scale. J Korean Neuropsychiatr Assoc. 2005; 44(4):466–476.
13. Trenkwalder C, Kohnen R, Högl B, Metta V, Sixel-Döring F, Frauscher B, et al. Parkinson's disease sleep scale--validation of the revised version PDSS-2. Mov Disord. 2011; 26(4):644–652.

14. Kwon DY, Kim JW, Ma HI, Ahn TB, Cho J, Lee PH, et al. Translation and validation of the Korean version of the 39-item Parkinson's disease questionnaire. J Clin Neurol. 2013; 9(1):26–31.

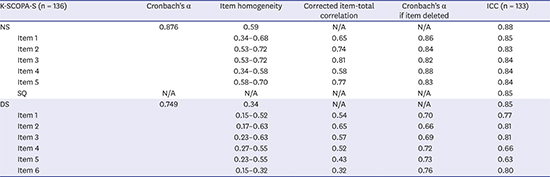
15. Koh SB, Kim JW, Ma HI, Ahn TB, Cho JW, Lee PH, et al. Validation of the Korean-version of the nonmotor symptoms scale for Parkinson's disease. J Clin Neurol. 2012; 8(4):276–283.

16. Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007; 22(16):2386–2393.

17. Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012; 22(2):79–90.

18. Setthawatcharawanich S, Limapichat K, Sathirapanya P, Phabphal K. Validation of the Thai SCOPA-sleep scale for assessment of sleep and sleepiness in patients with Parkinson's disease. J Med Assoc Thai. 2011; 94(2):179–184.
19. Hagell P, Westergren A, Janelidze S, Hansson O. The Swedish SCOPA-SLEEP for assessment of sleep disorders in Parkinson's disease and healthy controls. Qual Life Res. 2016; 25(10):2571–2577.

20. Martinez-Martin P, Visser M, Rodriguez-Blazquez C, Marinus J, Chaudhuri KR, van Hilten JJ, et al. SCOPA-sleep and PDSS: two scales for assessment of sleep disorder in Parkinson's disease. Mov Disord. 2008; 23(12):1681–1688.

21. Scaravilli T, Gasparoli E, Rinaldi F, Polesello G, Bracco F. Health-related quality of life and sleep disorders in Parkinson's disease. Neurol Sci. 2003; 24(3):209–210.

22. Borek LL, Kohn R, Friedman JH. Mood and sleep in Parkinson's disease. J Clin Psychiatry. 2006; 67(6):958–963.

23. Verbaan D, van Rooden SM, Visser M, Marinus J, van Hilten JJ. Nighttime sleep problems and daytime sleepiness in Parkinson's disease. Mov Disord. 2008; 23(1):35–41.

24. Damiano AM, Snyder C, Strausser B, Willian MK. A review of health-related quality-of-life concepts and measures for Parkinson's disease. Qual Life Res. 1999; 8(3):235–243.
25. Karlsen KH, Larsen JP, Tandberg E, Maeland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999; 66(4):431–435.

26. You S, Cho YW. Sleep disorders in patients with Parkinson's disease. J Korean Sleep Res Soc. 2014; 11(2):45–49.

27. Suzuki K, Miyamoto M, Miyamoto T, Okuma Y, Hattori N, Kamei S, et al. Correlation between depressive symptoms and nocturnal disturbances in Japanese patients with Parkinson's disease. Parkinsonism Relat Disord. 2009; 15(1):15–19.

28. Arnao V, Cinturino A, Valentino F, Perini V, Mastrilli S, Bellavia G, et al. In patient's with Parkinson disease, autonomic symptoms are frequent and associated with other non-motor symptoms. Clin Auton Res. 2015; 25(5):301–307.

29. Zhou J, Zhang J, Lam SP, Chan JW, Mok V, Chan A, et al. Excessive daytime sleepiness predicts neurodegeneration in idiopathic REM sleep behavior disorder. Sleep. 2017; 40(5):zsx041.

30. Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, Nithi K, Talbot K, Ben-Shlomo Y, et al. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson's disease. J Neurol Neurosurg Psychiatry. 2014; 85(5):560–566.

31. Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Mov Disord. 2012; 27(6):720–726.

32. Pushpanathan ME, Loftus AM, Thomas MG, Gasson N, Bucks RS. The relationship between sleep and cognition in Parkinson's disease: a meta-analysis. Sleep Med Rev. 2016; 26:21–32.

33. Kang SH, Lee HM, Seo WK, Kim JH, Koh SB. The combined effect of REM sleep behavior disorder and hyposmia on cognition and motor phenotype in Parkinson's disease. J Neurol Sci. 2016; 368:374–378.

34. Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002; 73(6):629–635.

35. Martínez-Martín P, Salvador C, Menéndez-Guisasola L, González S, Tobías A, Almazán J, et al. Parkinson's Disease Sleep Scale: validation study of a Spanish version. Mov Disord. 2004; 19(10):1226–1232.

36. Martínez-Martín P, Cubo-Delgado E, Aguilar-Barberà M, Bergareche A, Escalante S, Rojo A, et al. A pilot study on a specific measure for sleep disorders in Parkinson's disease: SCOPA-Sleep. Rev Neurol. 2006; 43(10):577–583.
37. Young A, Home M, Churchward T, Freezer N, Holmes P, Ho M. Comparison of sleep disturbance in mild versus severe Parkinson's disease. Sleep. 2002; 25(5):573–577.

38. Happe S, Lüdemann P, Berger K; FAQT study investigators. The association between disease severity and sleep-related problems in patients with Parkinson's disease. Neuropsychobiology. 2002; 46(2):90–96.

39. Gjerstad MD, Wentzel-Larsen T, Aarsland D, Larsen JP. Insomnia in Parkinson's disease: frequency and progression over time. J Neurol Neurosurg Psychiatry. 2007; 78(5):476–479.










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download