Abstract
Purpose
To evaluate whether early visual acuity response at 4 weeks after the first intravitreal anti-vascular endothelial growth factor (VEGF) injection or 4 weeks after the third injection in neovascular age-related macular degeneration (nAMD) is associated with 12-month follow-up outcome.
Methods
Thirty treatment-naive patients (30 eyes) with nAMD, treated with intravitreal anti-VEGF, were retrospectively included. Initially, all patients were injected at least three times for three consecutive months and followed up with a pro re nata regimen for at least 12 months. The relationship between 4 weeks after the first and third anti-VEGF injections in visual acuity response was explored, including the mean change from baseline in best-corrected visual acuity (BCVA). The mean change in BCVA was classified into three groups according to visual improvement: <1, 1‒<3, or ≥ 3 logMAR line(s) in BCVA. The associations among baseline characteristics (gender, age, duration of symptoms, initial BCVA, central macular thickness, and intraocular pressure) and visual acuity responses 4 weeks after the first and third anti-VEGF injections were also assessed.
Results
The proportions of eyes with <1, 1–<3, and ≥ 3-line(s) improvement at 4 weeks after the first injection were 6 eyes (20%), 7 eyes (23.3%), and 17 eyes (56.6%), respectively. The proportions of eyes with <1, 1–<3, and ≥ 3-line(s) improvement at 4 weeks after the third injection were 9 eyes (30%), 9 eyes (30%), and 12 eyes (40%), respectively. A BCVA response ≥3-lines improvement at 4 weeks after the third injection showed significant associations with ≥ 3-lines improvement and BCVA response at 12 months in multiple logistic and linear regression analyses (p = 0.04).
Go to : 
References
1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-abdominal macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.
2. Au Eong KG. Age-related macular degeneration: an emerging challenge for eye care and public health professionals in the Asia Pacific region. Ann Acad Med Singapore. 2006; 35:133–5.
3. Park SJ, Lee JH, Woo SJ, et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014; 121:1756–65.
4. Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002; 99:11393–8.

5. Brechner RJ, Rosenfeld PI, Babish JD, et al. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. Am J Ophthalmol. 2011; 151:887–95.e1.

6. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus abdominal for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
7. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

8. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.

9. Ying GS, Maguire MG, Daniel E, et al. Association of baseline characteristics and early vision response with 2-year vision abdominals in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2015; 122:2523–31.e1.
10. Sagiv O, Zloto O, Moroz I, Moisseiev J. Different clinical courses on long-term follow-up of age-related macular degeneration abdominals treated with intravitreal anti-vascular endothelial growth abdominal injections. Ophthalmologica. 2017; 238:217–25.
11. Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal recurrent neovascular lesions in age related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991; 109:1232–41.
12. Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular abdominal: updated findings from two clinical trials. Arch Ophthalmol. 1993; 111:1200–9.
13. Zhang X, Lai TYY. Baseline predictors of visual acuity outcome in patients with wet age-related macular degeneration. Biomed Res Int. 2018; 2018:9640131.

14. Lee HW, Kim HC. Correlation between visual outcomes and pre-treatment factors including hyperreflective foci in neovascular age-related macular degeneration. J Korean Ophthalmol Soc. 2015; 56:1188–94.
15. Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and abdominal tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012; 96:14–20.
16. Ehlken C, Jungmann S, Böhringer D, et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye (Lond). 2014; 28:538–45.

17. Kaiser PK. Registry of Visudyne AMD Therapy Writing Committee. Boyer DS, et al. Verteporfin photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2009; 116:747–55.

18. Kaiser PK, Boyer DS, Cruess AF, et al. Verteporfin plus abdominal for choroidal neovascularization in age-related macular abdominal: twelve-month results of the DENALI study. Ophthalmology. 2012; 119:1001–10.
19. Larsen M, Schmidt-Erfurth U, Lanzetta P, et al. Verteporfin plus abdominal for choroidal neovascularization in age-related macular abdominal: twelve-month MONT BLANC study results. Ophthalmology. 2012; 119:992–1000.
Go to : 
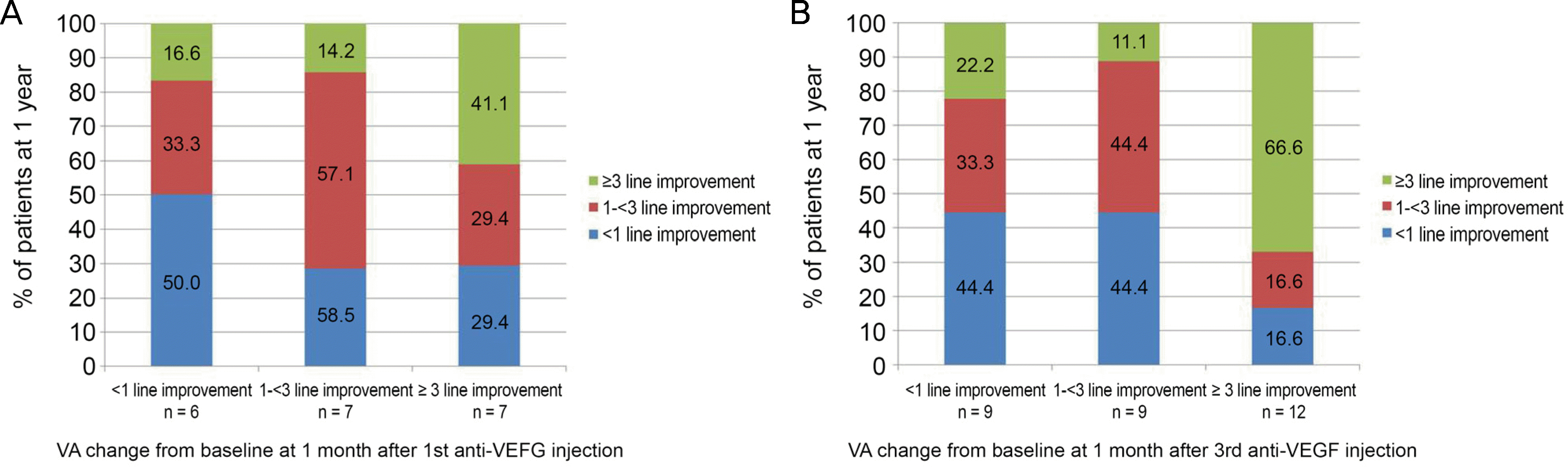 | Figure 1.The visual acuity (VA) response category (≥3 line improvement, 1-<3 line improvement, <1 line improvement) at year 1 by the early VA response category at 1 month after 1st anti-VEGF injection or at 1 month after 3rd anti-VEGF injection. VA response category at 1 year by VA response at 1 month after 1st anti-VEGF injection (A), and VA response category at 1 year by VA response at 1 month after 3rd anti-VEGF injection (B). VEGF = vascular endothelial growth factor. |
Table 1.
Distribution of initial 3 consecutive intravitreal anti-VEGF injections, categorized by additional anti-VEGF injections
Table 2.
Demographics and baseline characteristics of pooled study eyes, categorized by BCVA response at 1 month after the 1st an-ti-VEGF injection
|
BCVA changes from baseline at 1 month after the first anti-VEGF injection |
p-value | |||
|---|---|---|---|---|
| <1 line improvement (n = 6) | 1-<3 line improvement (n = 7) | ≥3 line improvement (n = 17) | ||
| Gender (male/female) | 4/2 | 4/3 | 10/7 | 0.78* |
| Age (years) | 68.50 ± 8.31 | 72.85 ± 5.84 | 74.17 ± 4.79 | 0.14† |
| Duration of symptom (months) | 2.00 ± 0.89 | 2.42 ± 1.13 | 1.70 ± 0.68 | 0.17† |
| Baseline BCVA (logMAR) | 0.56 ± 0.16 | 0.56 ± 0.13 | 0.57 ± 0.16 | 0.61†‡ |
| Baseline CMT (μ m) | 387.11 ± 52.93 | 407.35 ± 42.34 | 422.10 ± 33.42 | 0.35† |
| IOP (mmHg) | 12.72 ± 3.43 | 13.21 ± 1.62 | 12.94 ± 2.15 | 0.47† |
Table 3.
Demographics and baseline characteristics of pooled study eyes, categorized by BCVA response at 1 month after the 3rd an-ti-VEGF injection
|
BCVA changes from baseline at 1 month after the third anti-VEGF injection |
p-value | |||
|---|---|---|---|---|
| <1 line improvement (n = 9) | 1-<3 line improvement (n = 9) | ≥3 line improvement (n = 12) | ||
| Gender (male/female) | 5/3 | 6/3 | 7/6 | 0.16* |
| Age (years) | 74.11 ± 5.75 | 73.00 ± 6.24 | 71.50 ± 6.38 | 0.73† |
| Duration of symptom (months) | 1.55 ± 0.52 | 1.88 ± 0.92 | 2.16 ± 0.93 | 0.29† |
| Baseline BCVA (logMAR) | 0.34 ± 0.08 | 0.51 ± 0.07 | 0.63 ± 0.06 | 0.11†‡ |
| Baseline CMT (μ m) | 418.11 ± 25.47 | 415.00 ± 41.48 | 402.08 ± 52.14 | 0.35† |
| IOP (mmHg) | 13.21 ± 3.33 | 12.43 ± 1.42 | 12.92 ± 2.22 | 0.53† |
Table 4.
Association of visual acuity response at 1 month after 1st anti-VEGF injection or at 1 month after 3rd anti-VEGF injection with visual acuity response at year 1
Table 5.
Logistic regression analysis of 3 lines or more visual improvement at 12 months
|
Logistic regression of ≥3 lines improvement in BCVA at 12 months |
||||
|---|---|---|---|---|
|
Univariated |
Multivariated |
|||
| Odds ratio | p-value* | Odds ratio | p-value* | |
| Age (years) | 0.05 | 0.72 | ||
| Male:Female | 2.22 | 0.45 | ||
| Baseline BCVA (logMAR) | 0.15 | 0.62 | ||
| Baseline CMT (μ m) | 0.99 | 0.43 | ||
| BCVA change ≥3 lines at 1 month after first anti-VEGF injection | 12.29 | 0.50 | 19.26 | 0.13 |
| BCVA change ≥3 lines at 1 month after third anti-VEGF injection | 886.54 | 0.04 | 25.66 | 0.02 |
| Cumulative number of anti-VEGF injection | 2.00 | 0.24 | ||
| Symptom duration | 0.53 | 0.24 | ||
Table 6.
Linear regression analysis of changes from baseline in BCVA at 12 months
|
Linear regression of BCVA response at 12 months |
||||
|---|---|---|---|---|
|
Univariated |
Multivariated |
|||
| Estimate* | p-value† | Estimate* | p-value‡ | |
| Age (years) | −0.06 | 0.75 | 0.03 | 0.18 |
| Male:Female | −0.23 | 0.21 | −0.13 | 0.48 |
| Baseline BCVA (logMAR) | 0.33 | 0.07 | 0.12 | 0.57 |
| Baseline CMT (μ m) | −0.97 | 0.60 | 0.01 | 0.84 |
| BCVA change ≥3 lines at 1 month after first anti-VEGF injection | 0.20 | 0.27 | 0.08 | 0.66 |
| BCVA change ≥3 lines at 1 month after third anti-VEGF injection | 0.58 | 0.01 | 0.52 | 0.02 |
| Cumulative number of anti-VEGF injection | 0.11 | 0.54 | 0.04 | 0.80 |
| Symptom duration | 0.14 | 0.44 | −0.09 | 0.61 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download