Abstract
Purpose
To investigate the effects of valproic acid on the survival of cultured human Tenon's capsule fibroblasts (HTFBs).
Methods
Primary cultured HTFBs were exposed to 0, 0.25, 0.5, and 1.0 mM valproic acid with or without 0, 1.0, 2.5 µg/mL mitomycin C, and incubated for 5 days. Cell survival was assessed using an MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay and the degree of apoptosis was assessed by flow cytometry using annexin-V/propidium iodide double staining.
Figures and Tables
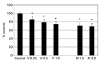 | Figure 1Effect of valproic acid and mitomycin C on the survival of human Tenon's capsule fibroblasts. Both valproic acid (V, mM) and mitomycin C (M, µg/mL) decreased cellular survival in a dose-dependent manner (*p < 0.05). |
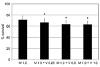 | Figure 2Additive effect of valproic acid (V, mM) on the survival of human Tenon's capsule fibroblasts when co-exposed to 1.0 µg/mL of mitomycin C (M). Valproic acid further decreased mitomycin C-induced reduction of cellular survival significantly (*p < 0.05). |
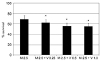 | Figure 3Additive effect of valproic acid (V, mM) on the survival of human Tenon's capsule fibroblasts when co-exposed to 2.5 µg/mL of mitomycin C (M). Valproic acid further decreased mitomycin C-induced reduction of cellular survival significantly (*p < 0.05). |
 | Figure 4Flow cytometric analysis of apoptosis using annexin-propidium iodide (PI) double staining. Cells in quadrant B1, B2, B3, B4 represent necrotic cells, late apoptotic cells, living cells and early apoptotic cells, respectively. (A) Exposed to 0.5 mM valproic acid. (B) Exposed to 2.5 µg/mL of mitomycin C. Cell count = 10,000. FITC = fluorescein isothiocyanate. |
References
1. Addicks EM, Quigley HA, Green WR, Robin AL. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983; 101:795–798.

2. Skuta GL, Parrish RK 2nd. Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987; 32:149–170.

3. Bindlish R, Condon GP, Schlosser JD, et al. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002; 109:1336–1341. discussion 1341-2.
4. Migdal C, Hitchings R. Morbidity following prolonged postoperative hypotony after trabeculectomy. Ophthalmic Surg. 1988; 19:865–867.

5. Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with mitomycin C. Am J Ophthalmol. 1993; 116:314–326.

6. Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003; 110:1185–1191.

7. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003; 48:314–346.

8. Kim SH, Kim JW. Comparison of the effects between bevacizumab and mitomycin C on the survival of fibroblasts. J Korean Ophthalmol Soc. 2011; 52:345–349.

9. Löescher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002; 16:669–694.
10. Monti B, Polazzi E, Contestabile A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol. 2009; 2:95–109.

11. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003; 37 Suppl 2:5–16.
12. Kawagoe R, Kawagoe H, Sano K. Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase-dependent and –independent apoptotic signaling pathway. Leuk Res. 2002; 26:495–502.
13. Phillips A, Bullock T, Plant N. Sodium valproate induces apoptosis in the rat hepatoma cell line, FaO. Toxicology. 2003; 192:219–227.

14. Tang R, Faussat AM, Majdak P, et al. Valproic acid inhibits proliferation and induces apoptosis in acute myeloid leukemia cells expressing P-gp and MRP1. Leukemia. 2004; 18:1246–1251.

15. Witt D, Burfeind P, von Hardenberg S, et al. Valproic acid inhibits the proliferation of cancer cells by re-expressing cyclin D2. Carcinogenesis. 2013; 34:1115–1124.

16. Michaelis M, Michaelis UR, Fleming I, et al. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol. 2004; 65:520–527.

17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

18. Biermann J, Grieshaber P, Goebel U, et al. Valproic acid–mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010; 51:526–534.

19. Göttlicher M, Minucci S, Zhu P, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001; 20:6969–6978.
20. Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001; 276:36734–36741.

21. Alsarraf O, Fan J, Dahrouj M, et al. Acetylation preserves retinal ganglion cell structure and function in a chronic model of ocular hypertension. Invest Ophthalmol Vis Sci. 2014; 55:7486–7493.

22. Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001; 276:36734–36741.

23. Chang KY, Moon JI, Baek NH, Lee CJ. The tissue changes of filtering site following glaucoma filtration surgery with various mitomycin C concentrations. J Korean Ophthalmol Soc. 1995; 36:316–323.
24. Mietz H, Addicks K, Bloch W, Krieglstein GK. Long-term intraocular toxic effects of topical mitomycin C in rabbits. J Glaucoma. 1996; 5:325–333.

25. Witt D, Burfeind P, von Hardenberg S, et al. Valproic acid inhibits the proliferation of cancer cells by re-expressing cyclin D2. Carcinogenesis. 2013; 34:1115–1124.

26. Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002; 7:1–10.

27. Kim KH, Kim JW. Role of nitric oxide on the proliferation of human Tenon capsule fibroblasts. J Korean Ophthalmol Soc. 2003; 44:1670–1674.
28. Hyndman KA, Ho DH, Sega MF, Pollock JS. Histone deacetylase 1 reduces NO production in endothelial cells via lysine deacetylation of NO synthase 3. Am J Physiol Heart Circ Physiol. 2014; 307:H803–H809.

29. Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtering surgery. Invest Ophthalmol Vis Sci. 2009; 50:5217–5225.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download