Abstract
Purpose
A recently introduced phacoemulsification system, the WhiteStar Signature® PRO, has demonstrated improved nucleus followability and cutting efficiency via an improved pump regulator with a higher reaction response and an automatic occlusion sensing system. In this study, we compared various phacoemulsification parameters between the new system and an older version of the device.
Methods
A total of 80 eyes of 68 patients with cataracts who had undergone phacoemulsification by a single surgeon were included in this study. Forty eyes of patients underwent phacoemulsification using the older Signature® system (WhiteStar); these patients were classified as the control group. Another 40 eyes of patients underwent phacoemulsification with the newer enhanced system, the WhiteStar Signature® PRO; these patients were assigned to the experimental group. During the operation, operative parameters, including the effective phaco time (parameter of effective phaco time with a specific coefficient for the transversal movement expressed in seconds, EFX), ultrasound time (seconds [s]), effective phacoemulsification time (EPT, s), average phacoemulsification power (AVG, %), and balanced salt solution usage, were measured to determine the performance enhancement offered by the updated system. Central corneal thickness was measured before and after surgery to compare corneal edema. The relationships between the two groups were analyzed using an independent t-test.
Go to : 
References
1. Kelman CD. Phaco– emulsification and aspiration. a new technique of cataract removal. a preliminary report. Am J Ophthalmol. 1967; 64:23–35.
2. Haripriya A, Chang DF, Reena M, Shekhar M. Complication rates of phacoemulsification and manual small-incision cataract surgery at Aravind Eye Hospital. J Cataract Refract Surg. 2012; 38:1360–9.

3. Vargas LG, Holzer MP, Solomon KD, et al. Endothelial cell abdominal after phacoemulsification with 2 different handpieces. J Cataract Refract Surg. 2004; 30:478–82.
4. O'Brien PD, Fitzpatrick P, Kilmartin DJ, Beatty S. Risk factors for endothelial cell loss after phacoemulsification surgery by a junior resident. J Cataract Refract Surg. 2004; 30:839–43.
5. Kim DH, Wee WR, Lee JH, Kim MK. The comparison between torsional and conventional mode phacoemulsification in moderate and hard cataracts. Korean J Ophthalmol. 2010; 24:336–40.

6. Liu Y, Zeng M, Liu X, et al. Torsional mode versus conventional ultrasound mode phacoemulsification: randomized comparative clinical study. J Cataract Refract Surg. 2007; 33:287–92.
7. Georgescu D, Kuo AF, Kinard KI, Olson RJ. A fluidics comparison of Alcon Infiniti, Bausch & Lomb Stellaris, and Advanced Medical Optics Signature phacoemulsification machines. Am J Ophthalmol. 2008; 145:1014–7.
8. Schmutz JS, Olson RJ. Thermal comparison of Infiniti Ozil and Signature Ellips phacoemulsification systems. Am J Ophthalmol. 2010; 149:762–7.e1.

9. Chu YR, Mah FS, Tyson F, et al. Ins and outs. J Cataract Refract Surg 2016 Jan. https://crstoday.com/articles/2016-jan/ins-and-outs/. Accessed February 7, 2018.
10. Wright AJ, Thomson RS, Bernhisel AA, et al. Effect of chamber stabilization software on efficiency and chatter in a porcine lens model. J Cataract Refract Surg. 2017; 43:1464–7.

11. Sharif-Kashani P, Fanney D, Injev V. Comparison of occlusion break responses and vacuum rise times of phacoemulsification systems. BMC Ophthalmol. 2014; 14:96.

12. Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993; 111:831–6.
13. Ataş M, Demircan S, Karatepe Haş haş AS, et al. Comparison of corneal endothelial changes following phacoemulsification with transversal and torsional phacoemulsification machines. Int J Ophthalmol. 2014; 7:822–7.
14. Gwin RM, Warren JK, Samuelson DA, Gum GG. Effects of abdominal and extracapsular lens removal on corneal abdominal and endothelial cell density in the dog. Invest Ophthalmol Vis Sci. 1983; 24:227–36.
15. Tsinopoulos IT, Lamprogiannis LP, Tsaousis KT, et al. Surgical outcomes in phacoemulsification after application of a risk strat-ification system. Clin Ophthalmol. 2013; 7:895–9.

16. Meyer JJ, Kuo AF, Olson RJ. The risk of capsular breakage from phacoemulsification needle contact with the lens capsule: a abdominal study. Am J Ophthalmol. 2010; 149:882–6.e1.
17. Lee JE, Choi SH. Comparison of clinical results between Ellips and Ozil modes in phacoemulsification. J Korean Ophthalmol Soc. 2011; 52:1161–6.

18. Tognetto D, D'Aloisio R, Cecchini P, et al. Comparative clinical study of Whitestar Signature phacoemulsification system with standard and Ellips FX handpieces. Int Ophthalmol. 2018; 38:1697–702.

19. Walkow T, Anders N, Klebe S. Endothelial cell loss after abdominal: relation to preoperative and intraoperative parameters. J Cataract Refract Surg. 2000; 26:727–32.
20. Bourne RR, Minassian DC, Dart JK, et al. Effect of cataract abdominal on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004; 111:679–85.
21. Cameron MD, Poyer JF, Aust SD. Identification of free radicals produced during phacoemulsification. J Cataract Refract Surg. 2001; 27:463–70.

22. Joussen AM, Barth U, Cubuk H, Koch H. Effect of irrigating abdominal and irrigation temperature on the cornea and pupil during phacoemulsification. J Cataract Refract Surg. 2000; 26:392–7.
Go to : 
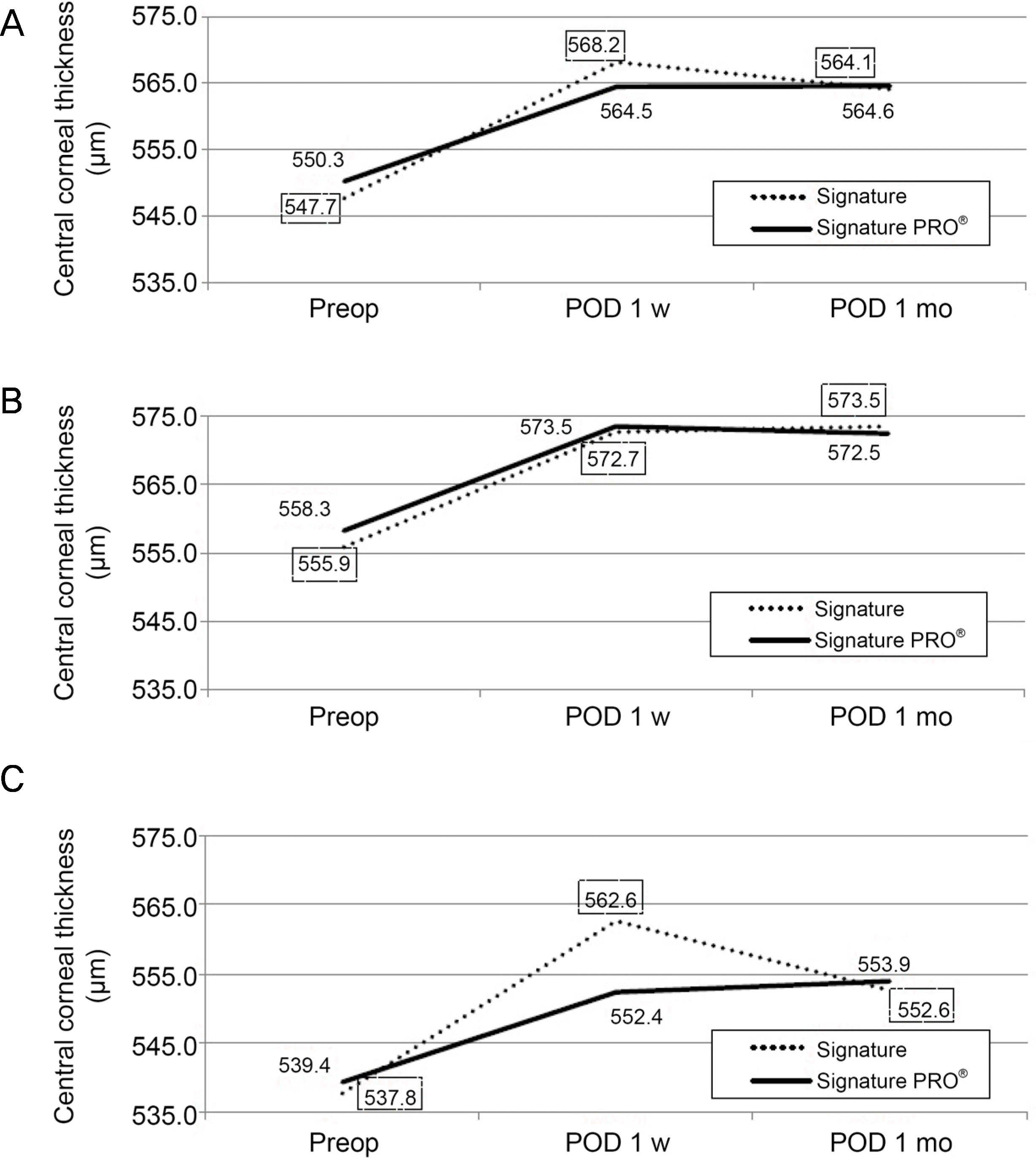 | Figure 1.Change of preoperative and post-operative central corneal thickness (CCT) (μ m). This graph features changes of CCT between two phacoemulsification systems. A solid line shows the mean CCT values in the group using enhanced device group (Signature PRO® system) and a dotted line shows the mean CCT values in the control group (Signature® system). Using independent t-test, there is no statistically significant difference between two systems (p > 0.05) in total and subgroup analysis. (A) Total group. (B) Soft nucleus subgroup. (C) Hard nucleus subgroup. Preop = pre-operative examination; POD = post-operative day; w= week; mo = month. |
Table 1.
Phacoemulsification settings in two systems
Table 2.
Demographics of preoperative conditions
| Control group (Signature® system) | Enhanced device group (Signature PRO® system) | p-value* | |
|---|---|---|---|
| Eyes (n) | 40 | 40 | |
| Age (year) | 68.40 ± 11.72 | 70.35 ± 10.00 | 0.425 |
| NO (LOCS III) | 3.44 ± 1.01 | 3.43 ± 1.08 | 0.957 |
| Axial length (mm) | 23.37 ± 1.34 | 23.78 ± 1.12 | 0.143 |
| Anterior chamber depth (mm) | 2.64 ± 0.49 | 2.65 ± 0.30 | 0.875 |
| Best corrected visual acuity (logMAR) | 0.37 ± 0.39 | 0.45 ± 0.58 | 0.483 |
| Endothelial cell count (cell/mm2) | 2,747.60 ± 312.72 | 2,599.10 ± 462.86 | 0.097 |
| Central corneal thickness (μ m) | 547.73 ± 28.70 | 550.25 ± 39.32 | 0.744 |
Table 3.
Comparisons of surgical parameters during phacoemulsification
| Control group (Signature® system) | Enhanced device group (Signature PRO® system) | p-value | |
|---|---|---|---|
| Surgical parameters | |||
| EFX | 2.11 ± 1.75 | 0.40 ± 0.61 | <0.001* |
| Ultrasonic/phaco time (seconds) | 58.79 ± 33.87 | 44.31 ± 42.22 | 0.095 |
| Effective phaco time (seconds) | 4.22 ± 3.49 | 0.80 ± 1.22 | <0.001* |
| Average phaco power (%) | 7.28 ± 4.03 | 1.23 ± 1.21 | <0.001* |
| Irrigated BSS (mL) | 95.75 ± 56.89 | 131.00 ± 52.84 | 0.005* |
Table 4.
Comparisons of surgical parameters during phacoemulsification in soft and hard nucleus group
|
Soft nucleus |
Hard nucleus |
|||||
|---|---|---|---|---|---|---|
| Control group (Signature® system) | Enhanced device group (Signature PRO® system) | p-value | Control group (Signature® system) | Enhanced device group (Signature PRO® system) | p-value | |
| Surgical parameters | ||||||
| EFX | 1.34 ± 0.86 | 0.13 ± 0.17 | <0.001* | 3.05 ± 2.09 | 0.77 ± 0.79 | <0.001* |
| Ultrasonic/phaco time (seconds) | 45.85 ± 31.04 | 28.71 ± 29.66 | 0.065 | 74.61 ± 30.97 | 7 65.43 ± 48.08 | 0.504 |
| Effective phaco time (seconds) | 2.68 ± 1.72 | 0.26 ± 0.35 | <0.001* | 6.11 ± 4.18 | 1.53 ± 1.57 | <0.001* |
| Average phaco power (%) | 7.28 ± 4.03 | 1.23 ± 1.21 | <0.001* | 8.06 ± 3.46 | 2.12 ± 1.27 | <0.001* |
| Irrigated BSS (mL) | 82.27 ± 36.15 | 124.13 ± 49.03 | 0.002* | 112.22 ± 72.72 | 2 140.29 ± 57.81 | 0.214 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download