Abstract
In this study, we report a case of anti-Gerbich (Ge) alloantibody to a high-prevalence Ge antigen in a donor with Fy(a−b−) phenotype. The alloantibody was detected in an Emirati boy who was admitted to a Korean tertiary hospital for marrow hematopoietic progenitor cell donation. He did not have a history of transfusion. His blood type was A, RhD+, and findings from the antibody screening and identification test showed 2+ reactivity in all panel cells except autologous cells. We concluded that it would be very difficult to find compatible blood components for the donor and requested further tests from external laboratories. Anti-Ge2 was identified by additional tests in a foreign reference laboratory, and the Duffy genotype of the donor was FY*02/FY*02N.01 based on the Korean Rare Blood Program. Although the donor was not a Korean, as the number of foreign patients visiting Korea increases annually, there is growing interest in patients with rare blood types in the Korean population. However, there has been very little research on rare or high prevalence blood type antigen and antibody in the Korean population. Therefore, additional research in Korea is needed on rare blood group antibodies and antigens, including Ge cases.
The Gerbich (Ge) blood group consists of antigens that are located on glycophorin C, glycophorin D, or both.1 There are six known high-prevalence antigens (Ge2, Ge3, Ge4, GEPL, GEAT, and GETI) and five low-prevalence antigens (Wb, Lsa, Ana, Dha, and GEIS).2 In Melanesians of Papua New Guinea, over 50% of the population is Ge-negative, and about 10% is reported to have natural anti-Ge.3 In general, however, Ge is a very common blood group system; therefore, it is considered to be extremely difficult to find compatible blood components for patients with anti-Ge.4
In this study, we report an Emirati boy who was admitted for marrow hematopoietic progenitor cell (HPC) donation to his sister. The donor had an irregular antibody identified as anti-Ge. In addition, his Duffy phenotype was Fy(a−b−), which is extremely rare in the Korean population.5
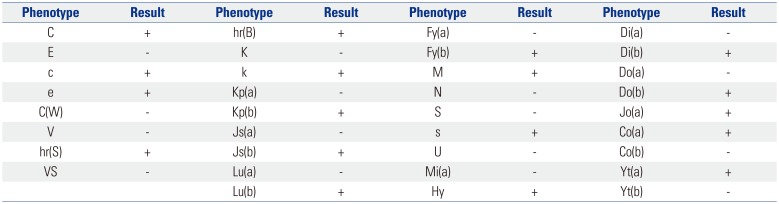
An 8-year-old Emirati boy was admitted to Severance Hospital as a marrow HPC donor for his sister in June 2017. He had no medical history. On admission, the results of physical examinations and laboratory tests including infectious disease testing showed that he was suitable for donation. His blood types were A, DCce, Le(a+b−), K−, Jk(a+b+), M+N−S−s+, and Fy(a−b−). The donor had no transfusion history. However, antibody screening test with ID-Diacell I, II (Bio-Rad, Cressier, Switzerland) and identification test (IDT) with ID-DiaPanel (Bio-Rad) using LISS/Coombs ID-card (Bio-Rad) based on the column agglutination test method showed moderate positivity (2+) in all three screening cells and 11 identification panel cells with negative reactions to autologous red blood cells (RBCs) in anti-human globulin phase. An IDT using enzyme (papain)-treated panel cells (ID-DiaPanel P, Bio-Rad) showed negative reactivities with all panel cells. The donor was suspected of harboring antibodies to high-prevalence antigens, and he showed Fy(a−b−) phenotype, which is extremely rare in Korean donors. Therefore, the donor donated two autologous RBC components before marrow harvest, because it could not be ruled out that the donor's antibody was anti-Fy3, which was expected to show incompatibility with almost all RBC components from Korean donors. The specimens from the donor with informed consent were sent to the Korean Rare Blood Program (KRBP) for genotyping of extended blood groups. The genotyping was performed using a Lifecodes RBC/RBC-R typing kit (Immucor, Norcross, GA, USA). The expected RBC phenotype based on genotyping is summarized in Table 1. These results were consistent with the phenotype tested using serologic methods, except the Duffy phenotype. Because the result of Duffy genotyping was FY*02/FY*02N.01 based on polymerase chain reaction–restriction fragment length polymorphism analysis, the expected Duffy phenotype of the donor was Fy(a−b+). In addition, the specimen of the donor was sent to the central laboratory of the Swiss Red Cross in Bern, Switzerland for further tests. In that laboratory, the anti-Ge antibody was identified. A negative result for Ge2 antigen was also shown using sera including anti-Ge2. The donor received two autologous RBC transfusions after successful marrow HPC collection, and the donor was discharged without requirement of allogeneic RBC transfusion during his hospitalization. This study was approved by the Institutional Review Board of Severance Hospital (approval number: 4-2018-0437).
Both immune-caused and naturally occurring anti-Ge have been reported, and a 61-year-old Caucasian male with anti-Ge alloantibody who had no transfusion history was reported previously.6 In our report, the donor did not have any previous RBC transfusions; therefore, the anti-Ge2 of the donor could be naturally occurring. Anti-Ge2 is not generally considered to be clinically significant, although anti-Ge3 causes mild to moderate hemolytic transfusion reactions and also causes hemolytic disease of the fetus and newborn.1 For the case in our report, the antibody of the donor could be identified as anti-Ge2, considering that Ge3 antigen is resistant to papain treatment, whereas Ge2 and Ge4 are sensitive and the reactivity with papain-treated panel cells disappeared in this case.1 The Ge2 phenotype of the donor was negative. In a previous study, more than 90 ABO-identical RBC units were performed crossmatch tests, and none of them were found to be compatible to a Saudi Arabian patient with anti-Ge2.4 However, a patient with strongly reactive anti-Ge2 received a transfusion of 4 units of Ge2-positive RBCs that was well tolerated, and his hemoglobin level increased adequately.7 Therefore, allogeneic RBC transfusion to the donor in our case may not be problematic.
In Korea, only one Korean female patient with anti-Ge was reported previously.8 The same foreign reference laboratory mentioned in our report confirmed the alloantibody. This is the second report of an anti-Ge case in Korea; however, the case in our report is not of a Korean patient, but of an Emirati patient. However, as the number (364189 from 186 countries in 2016) of foreign patients visiting Korea increases annually, there is growing interest in patients with rare blood types in the Korean population.9 Therefore, when a blood transfusion is needed in such cases, it can be difficult to prepare when the patient has a blood type that is not common in the Korean population, or if irregular antibodies to high-prevalence antigens in Koreans are identified in the serum of patients. However, identification of unknown alloantibodies in both cases reported in Korea (previous report8 and our report) was performed in a foreign country, and the result of the anti-Ge in our case was known after the donor was discharged. Therefore, active attempts are needed to establish a national standard reference laboratory in such areas.
The Duffy genotype of the donor was identified as FY*02/FY*02N.01. The Fy(a−b−) phenotype occurs in over 63% of African populations. This is caused by homozygosity for the FY*2 (or FY*B) allele carrying a c.1-67T>C in the GATA-1 promoter region, which prevents Fyb expression in RBCs. However, Duffy antigens are expressed in non-erythroid tissues, and Africans with the Fy(a−b−) phenotype rarely produce anti-Fyb or anti-Fy3.10 In this case, there was a discrepancy between the results of Duffy phenotype and genotype. This may be caused by the characteristics of Duffy antigen, zygosity (or dosage effect). Red cells from individuals who are heterozygous for the Duffy gene may express fewer antigens and, therefore, may be weakly reactive or nonreactive with a weak example of the corresponding antibody.11 Additionally, it was reported that individuals with the same genotype as that in our case could show the Fy(a−b−) phenotype.12 In this setting, phenotyping with antiserum reagents of different lot number from the same manufacturer or those from different manufacturers may be helpful to confirm phenotypes. Unfortunately, we did not have access to different anti-Fyb antiserum reagents, and therefore, we could not further evaluate the Duffy phenotype of the donor in our case. According to genetic testing results, the Duffy blood group did not need to be considered to identify compatible RBC units in our case. However, the genetic testing results were also known after the donor was discharged. As mentioned above, we suggest that more support is needed for a national standard reference laboratory.
Fortunately, there were no requirements for allogeneic RBC transfusions in the previous report8 and our report. However, it seems to be extremely difficult to find compatible RBC components in such settings in Korea. In this setting, we have to consider autologous RBC transfusion or allogeneic RBC transfusion from family members because it is expected that family members are likely to have compatible antigen types. However, those transfusion strategies cannot be a solution in all cases. Therefore, KRBP, a national rare blood program, was established in 2013 to attain an efficient supply of rare blood products. For this purpose, KRBP has investigated the frequency of rare blood groups in Koreans and has reported RBC genotyping for blood groups, including Rh, MNS, Kell, Duffy, Kidd, Lutheran, Diego, Cartwright, Scianna, Dombrock, Colton, Landsteiner-Wiener, Cromer, and Knops, in 419 Korean blood donors.13 However, RBC genotyping for the other blood groups including Ge cannot be performed by the KRBP. Therefore, more national support is needed to ensure that the KRBP has the capacity to test for more rare blood groups and irregular antibodies to those antigens. In addition, cryopreservation of blood represents a possible solution to problems of transfusions in such settings like our report. For this reason, establishment of a national frozen blood storage system for management of rare blood supply will be required in Korea.14
In conclusion, anti-Ge2 alloantibody was identified from an Emirati boy in a Korean hospital. Fortunately, the donor did not require allogeneic RBC transfusions. However, it remains extremely difficult to find compatible bloods in such settings in Korea. Moreover, there has been very little research on Ge blood type antigen and antibody in the Korean population. Therefore, additional research in Korea is needed on rare blood group antibodies and high prevalence antigens, including Ge cases.
ACKNOWLEDGEMENTS
The authors thank Yun Ji Hong, M.D., and Kyoung Un Park, M.D., KRBP (Korean Rare Blood Program) in the Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea, for blood group genotyping.
References
1. Fung MK, Eder AF, Spitalnik SL, Westhoff CM. Technical manual. 19th ed. Bethesda (MD): American Association of Blood Banks;2017. p. 338–339.
2. Jaskiewicz E, Peyrard T, Kaczmarek R, Zerka A, Jodlowska M, Czerwinski M. The Gerbich blood group system: old knowledge, new importance. Transfus Med Rev. 2018; 32:111–116. PMID: 29540278.

3. Booth PB, McLoughlin K. The Gerbich blood group system, especially in Melanesians. Vox Sang. 1972; 22:73–84. PMID: 5011657.

4. Singh RP. Antibodies against high frequency Gerbich 2 antigen (anti-Ge2): a real challenge in cross matching lab. Asian J Transfus Sci. 2013; 7:88–89. PMID: 23559777.

5. Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, et al. The global distribution of the Duffy blood group. Nat Commun. 2011; 2:266. PMID: 21468018.

6. McLoughlin K, Rogers J. Anti-Ge-a in an untransfused New Zealand male. Vox Sang. 1970; 19:94–96. PMID: 5488663.
7. Hildebrandt M, Hell A, Etzel F, Genth R, Salama A. Determination and successful transfusion of anti-Gerbich-positive red blood cells in a patient with a strongly reactive anti-Gerbich antibody. Infusionsther Transfusionsmed. 2000; 27:154–156. PMID: 10878485.

8. Jeon YL, Park TS, Cho SY, Oh SH, Kim MH, Kang SY, et al. The first Korean case report of anti-Gerbich. Ann Lab Med. 2012; 32:442–444. PMID: 23130346.

9. Korea Health Industry Development Institute. Statistics on international patients in Korea. 2016. accessed on 2018 June 25. Available at: https://www.khidi.or.kr/board/view?linkId=217289&menuId=MENU00100.
10. Lopez GH, Morrison J, Condon JA, Wilson B, Martin JR, Liew YW, et al. Duffy blood group phenotype-genotype correlations using high-resolution melting analysis PCR and microarray reveal complex cases including a new null FY*A allele: the role for sequencing in genotyping algorithms. Vox Sang. 2015; 109:296–303. PMID: 25900316.
11. Fung MK, Eder AF, Spitalnik SL, Westhoff CM. Technical manual. 19th ed. Bethesda (MD): American Association of Blood Banks;2017. p. 350.
12. Parasol N, Reid M, Rios M, Castilho L, Harari I, Kosower NS. A novel mutation in the coding sequence of the FY*B allele of the Duffy chemokine receptor gene is associated with an altered erythrocyte phenotype. Blood. 1998; 92:2237–2243. PMID: 9746760.

13. Hong YJ, Chung Y, Hwang SM, Park JS, Kwon JR, Choi YS, et al. Genotyping of 22 blood group antigen polymorphisms and establishing a national recipient registry in the Korean population. Ann Hematol. 2016; 95:985–991. PMID: 27021300.

Table 1
Expected Red Blood Cell Phenotype of an Emirati Marrow Hematopoietic Cell Donor Based on Genotyping from the Korean Rare Blood Program




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download