1. Song KJ, Kim JB, Kim J, Kim C, Park SY, Lee CH, et al. Part 2. Adult basic life support: 2015 Korean Guidelines for Cardiopulmonary Resuscitation. Clin Exp Emerg Med. 2016; 3(Suppl):S10–S16. PMID:
27752642.

2. Travers AH, Perkins GD, Berg RA, Castren M, Considine J, Escalante R, et al. Part 3: Adult basic life support and automated external defibrillation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation. 2015; 132(16 Suppl 1):S51–S83. PMID:
26472859.

3. Perkins GD, Handley AJ, Koster RW, Castrén M, Smyth MA, Olasveengen T, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation. 2015; 95:81–99. PMID:
26477420.
4. Brenner BE, Van DC, Cheng D, Lazar EJ. Determinants of reluctance to perform CPR among residents and applicants: the impact of experience on helping behavior. Resuscitation. 1997; 35:203–211. PMID:
10203397.

5. Hew P, Brenner B, Kaufman J. Reluctance of paramedics and emergency medical technicians to perform mouth-to-mouth resuscitation. J Emerg Med. 1997; 15:279–284. PMID:
9258774.

6. Yeh ST, Cawley RJ, Aune SE, Angelos MG. Oxygen requirement during cardiopulmonary resuscitation (CPR) to effect return of spontaneous circulation. Resuscitation. 2009; 80:951–955. PMID:
19520479.

7. Dorph E, Wik L, Strømme TA, Eriksen M, Steen PA. Oxygen delivery and return of spontaneous circulation with ventilation: compression ratio 2:30 versus chest compressions only CPR in pigs. Resuscitation. 2004; 60:309–318. PMID:
15050764.
8. Botran M, Lopez-Herce J, Urbano J, Solana MJ, Garcia A, Carrillo A. Chest compressions versus ventilation plus chest compressions: a randomized trial in a pediatric asphyxial cardiac arrest animal model. Intensive Care Med. 2011; 37:1873–1880. PMID:
21847647.

9. Fenici P, Idris AH, Lurie KG, Ursella S, Gabrielli A. What is the optimal chest compression-ventilation ratio? Curr Opin Crit Care. 2005; 11:204–211. PMID:
15928467.

10. Hostler D, Guimond G, Callaway C. A comparison of CPR delivery with various compression-to-ventilation ratios during two-rescuer CPR. Resuscitation. 2005; 65:325–328. PMID:
15919570.

11. Hüpfl M, Selig HF, Nagele P. Chest-compression-only versus standard cardiopulmonary resuscitation: a meta-analysis. Lancet. 2010; 376:1552–1557. PMID:
20951422.

12. Kleinman ME, Brennan EE, Goldberger ZD, Swor RA, Terry M, Bobrow BJ, et al. Part 5: Adult basic life support and cardiopulmonary resuscitation quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015; 132(18 Suppl 2):S414–S435. PMID:
26472993.
13. Yannopoulos D, Tang W, Roussos C, Aufderheide TP, Idris AH, Lurie KG. Reducing ventilation frequency during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respir Care. 2005; 50:628–635. PMID:
15871757.
14. Aufderheide TP, Sigurdsson G, Pirrallo RG, Yannopoulos D, McKnite S, von Briesen C, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004; 109:1960–1965. PMID:
15066941.

15. Spoormans I, Van Hoorenbeeck K, Balliu L, Jorens PG. Gastric perforation after cardiopulmonary resuscitation: review of the literature. Resuscitation. 2010; 81:272–280. PMID:
20064683.

16. Hwang SO, Kim SH, Kim H, Jang YS, Zhao PG, Lee KH, et al. Comparison of 15:1, 15:2, and 30:2 compression-to-ventilation ratios for cardiopulmonary resuscitation in a canine model of a simulated, witnessed cardiac arrest. Acad Emerg Med. 2008; 15:183–189. PMID:
18275449.

17. Cha KC, Kim YW, Kim TH, Jung WJ, Yook H, Choi E, et al. Comparison between 30:1 and 30:2 compression-to-ventilation ratios for cardiopulmonary resuscitation: are two ventilations necessary? Acad Emerg Med. 2015; 22:1261–1266. PMID:
26470011.

18. Berg RA, Otto CW, Kern KB, Sanders AB, Hilwig RW, Hansen KK, et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med. 1994; 22:282–290. PMID:
8306688.

19. Hamel MB, Phillips R, Teno J, Davis RB, Goldman L, Lynn J, et al. Cost effectiveness of aggressive care for patients with nontraumatic coma. Crit Care Med. 2002; 30:1191–1196. PMID:
12072667.

20. Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015; 132(18 Suppl 2):S465–S482. PMID:
26472996.
21. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008; 118:2452–2483. PMID:
18948368.
22. Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, et al. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013; 128:417–435. PMID:
23801105.
23. Sanders AB, Kern KB, Berg RA, Hilwig RW, Heidenrich J, Ewy GA. Survival and neurologic outcome after cardiopulmonary resuscitation with four different chest compression-ventilation ratios. Ann Emerg Med. 2002; 40:553–562. PMID:
12447330.

24. Lurie KG, Zielinski T, McKnite S, Aufderheide T, Voelckel W. Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation. 2002; 105:124–129. PMID:
11772887.

25. Dorph E, Wik L, Strømme TA, Eriksen M, Steen PA. Quality of CPR with three different ventilation:compression ratios. Resuscitation. 2003; 58:193–201. PMID:
12909382.

26. Cavus E, Meybohm P, Bein B, Steinfath M, Pöppel A, Wenzel V, et al. Impact of different compression-ventilation ratios during basic life support cardiopulmonary resuscitation. Resuscitation. 2008; 79:118–124. PMID:
18586375.

27. Kwak SJ, Kim YM, Baek HJ, Kim SH, Yim HW. Chest compression quality, exercise intensity, and energy expenditure during cardiopulmonary resuscitation using compression-to-ventilation ratios of 15:1 or 30:2 or chest compression only: a randomized, crossover manikin study. Clin Exp Emerg Med. 2016; 3:148–157. PMID:
27752633.

28. Menegazzi JJ, Check BD. Spontaneous agonal respiration in a swine model of out-of-hospital cardiac arrest. Acad Emerg Med. 1995; 2:1053–1056. PMID:
8597915.


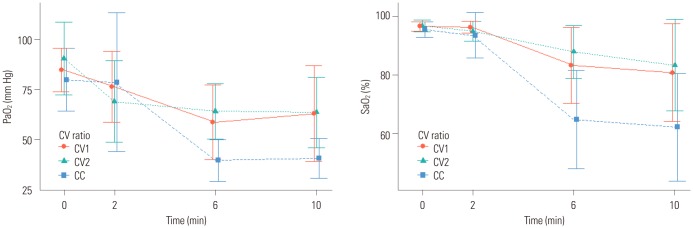
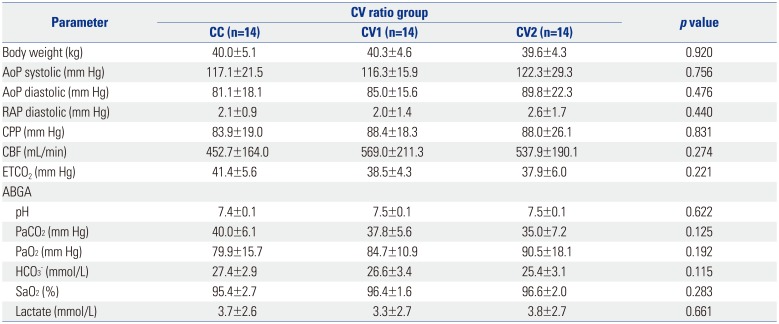
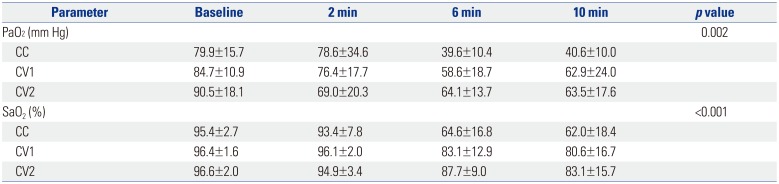
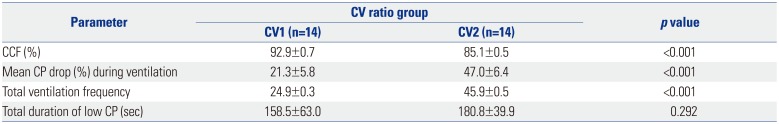




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download