Red blood cell (RBC) alloantibodies are formed following exposure to RBC alloantigens through transfusion, pregnancy, or transplantation [
1]. RBC alloimmunization increases the risk of a hemolytic transfusion reaction and makes it difficult to find compatible blood [
2]. Alloimmunization is more common in patients who require chronic transfusions, those with sickle cell anemia and thalassemia, and those with hematologic malignancies, especially myelodysplastic syndrome (MDS) [
3]. Several studies have sought to identify the risk factors for RBC alloimmunization, suggesting that alloimmunization is influenced by clinical condition, inflammatory status, genetic factors, gender, age, number of RBC units transfused, and immunogenicity of exposed antigens [
456].
When assessing the risk of alloimmunization to RBCs, commonly occurring diseases in the countries of interest should be considered. MDS and liver cirrhosis (LC) are representative diseases requiring chronic transfusion in Koreans [
7]. MDS, characterized by cytopenias associated with ineffective hematopoiesis, is usually treated with disease-modifying therapies and supportive care, such as transfusion [
8]. In advanced liver diseases, such as LC, bleeding due to esophageal varices occurs in up to 25% of patients and can be further aggravated by multiple coagulation abnormalities associated with liver dysfunction [
9]. However, there is no data on RBC alloimmunization in Korean patients with MDS and LC. Therefore, we evaluated the characteristics of RBC alloimmunization and compared the risk of alloimmunization in Korean MDS and LC patients.
We retrospectively reviewed medical records of MDS and LC patients transfused with RBCs at Samsung Medical Center, Seoul, Korea. This study was approved by the Institutional Review Board of Samsung Medical Center. Among patients transfused with RBCs between January 2013 and December 2015, a list of patients diagnosed as having MDS or LC was obtained from the electronic medical records. Patients displaying alloantibodies before the first RBC transfusion at the center or with no follow-up antibody screening test (AST) results were excluded. Initial AST was carried out with RBC panels from the Galileo NEO (Immucor Gamma, Norcross, GA, USA) or from QWALYS 3 (Diagast, Loos, France). When an antibody was detected, the AST was reiterated by a LISS/Coombs gel assay using ID-DiaCell I, II (Bio-Rad, Cressier-sur-Morat, Switzerland), and then, antibody specificity was identified using the ID-DiaPanel (Bio-Rad). If antibodies of undetermined specificity were found, they were additionally identified by the tube technique using the RESOLVE Panel A (Ortho-Clinical Diagnostics, Raritan, NJ, USA). Alloimmunization was defined as the positive conversion of AST after RBC transfusion in patients with initial negative AST. Follow-up duration of AST was defined as the period between the initial negative AST and follow-up positive AST in alloimmunized patients, and the period between the initial and last negative AST in non-alloimmunized patients. The number of transfused RBC units was recorded during the follow-up of AST.
Data were summarized as median with interquartile range (IQR) or as numbers and percentages. Patient characteristics were compared according to alloimmunization status, using the Mann–Whitney, chi-square, or Fisher's exact test. RBC alloimmunization rates were compared between the MDS and LC patients using the chi-square test. Cumulative risk of alloimmunization was estimated using a Kaplan–Meier plot with alloimmunization as the event and the cumulative number of transfused RBC units as the time variable. Alloimmunization risk was compared using the log-rank test. All statistical analyses were performed using IBM SPSS Statistics version 21 (IBM Corp., Armonk, NY, USA). P<0.05 was considered statistically significant.
Among the 133 MDS patients and 254 LC patients, 18 MDS and 52 LC patients were excluded for the following reasons: three MDS and nine LC patients were positive for initial AST, and 15 MDS and 43 LC patients did not undergo follow-up AST after RBC transfusion. Finally, 115 MDS patients and 202 LC patients were included (median age: 63 years; IQR: 54–72 years). The median number of transfused RBC units and the median duration of follow-up in AST were eight units (IQR: 3–24 units) and 102 days (IQR: 12–434 days), respectively. Patients received pre-storage leukocyte-reduced RBCs obtained from the Korea Red Cross blood center, leukocyte-depleted RBCs prepared in Samsung Medical Center, or general RBCs as clinically ordered. None of the patients had received antigen-matched RBC units before the RBC transfusion in this study.
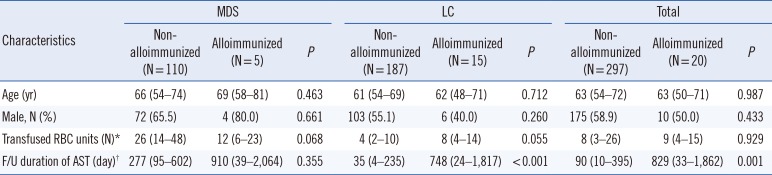
There were 20 newly alloimmunized patients among the 317 patients (6.3%) (
Table 1). The median number of RBC units transfused in alloimmunized patients was nine, which was not significantly different from that in non-alloimmunized patients (eight units;
P=0.929).
Table 1
Characteristics of MDS and LC patients
|
Characteristics |
MDS |
LC |
Total |
|
Non-alloimmunized (N = 110) |
Alloimmunized (N = 5) |
P
|
Non-alloimmunized (N = 187) |
Alloimmunized (N = 15) |
P
|
Non-alloimmunized (N = 297) |
Alloimmunized (N = 20) |
P
|
|
Age (yr) |
66 (54–74) |
69 (58–81) |
0.463 |
61 (54–69) |
62 (48–71) |
0.712 |
63 (54–72) |
63 (50–71) |
0.987 |
|
Male, N (%) |
72 (65.5) |
4 (80.0) |
0.661 |
103 (55.1) |
6 (40.0) |
0.260 |
175 (58.9) |
10 (50.0) |
0.433 |
|
Transfused RBC units (N)*
|
26 (14–48) |
12 (6–23) |
0.068 |
4 (2–10) |
8 (4–14) |
0.055 |
8 (3–26) |
9 (4–15) |
0.929 |
|
F/U duration of AST (day)†
|
277 (95–602) |
910 (39–2,064) |
0.355 |
35 (4–235) |
748 (24–1,817) |
< 0.001 |
90 (10–395) |
829 (33–1,862) |
0.001 |

With regard to disease, alloantibodies were observed in five (4.3%) and 15 (7.4%) MDS and LC patients, respectively (
P=0.278), and the median transfused RBC units (MDS vs. LC; 12 vs. 8 units;
P=0.405) did not differ. However, as the number of transfused RBC units increased, the cumulative risk of alloimmunization was higher in LC than in MDS patients (log-rank
P=0.001) (
Fig. 1).
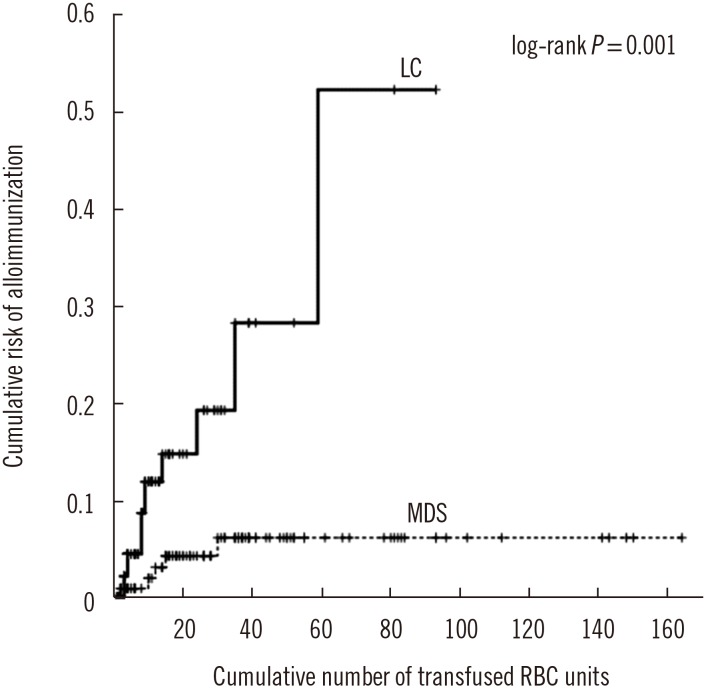 | Fig. 1
Kaplan–Meier plot for cumulative risk of alloimmunization in MDS and LC patients.
Abbreviations: MDS, myelodysplastic syndrome; LC, liver cirrhosis; RBC, red blood cell.

|
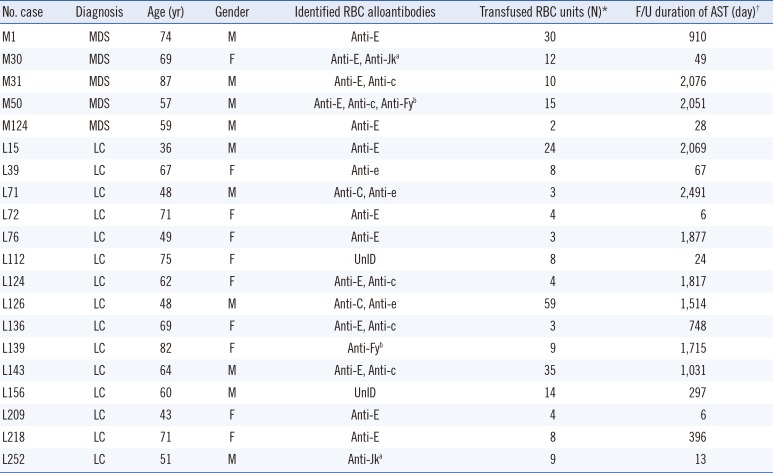
Of the 20 patients with alloantibodies, 10 (50%) had a single antibody and eight (40%) had multiple antibodies (
Table 2). Sixteen patients (80%) had at least one alloantibody of the Rh system. The most common alloantibody was anti-E (45%), followed by anti-c (17%), anti-e (10%), anti-C (7%), anti-Fy
b (7%), and anti-Jk
a (7%) (Supplemental Data Table S1). All of them were clinically significant alloantibodies that could cause hemolytic transfusion reactions [
10].
Table 2
Characteristics of alloimmunized patients and identified RBC alloantibodies
|
No. case |
Diagnosis |
Age (yr) |
Gender |
Identified RBC alloantibodies |
Transfused RBC units (N)*
|
F/U duration of AST (day)†
|
|
M1 |
MDS |
74 |
M |
Anti-E |
30 |
910 |
|
M30 |
MDS |
69 |
F |
Anti-E, Anti-Jka
|
12 |
49 |
|
M31 |
MDS |
87 |
M |
Anti-E, Anti-c |
10 |
2,076 |
|
M50 |
MDS |
57 |
M |
Anti-E, Anti-c, Anti-Fyb
|
15 |
2,051 |
|
M124 |
MDS |
59 |
M |
Anti-E |
2 |
28 |
|
L15 |
LC |
36 |
M |
Anti-E |
24 |
2,069 |
|
L39 |
LC |
67 |
F |
Anti-e |
8 |
67 |
|
L71 |
LC |
48 |
M |
Anti-C, Anti-e |
3 |
2,491 |
|
L72 |
LC |
71 |
F |
Anti-E |
4 |
6 |
|
L76 |
LC |
49 |
F |
Anti-E |
3 |
1,877 |
|
L112 |
LC |
75 |
F |
UnID |
8 |
24 |
|
L124 |
LC |
62 |
F |
Anti-E, Anti-c |
4 |
1,817 |
|
L126 |
LC |
48 |
M |
Anti-C, Anti-e |
59 |
1,514 |
|
L136 |
LC |
69 |
F |
Anti-E, Anti-c |
3 |
748 |
|
L139 |
LC |
82 |
F |
Anti-Fyb
|
9 |
1,715 |
|
L143 |
LC |
64 |
M |
Anti-E, Anti-c |
35 |
1,031 |
|
L156 |
LC |
60 |
M |
UnID |
14 |
297 |
|
L209 |
LC |
43 |
F |
Anti-E |
4 |
6 |
|
L218 |
LC |
71 |
F |
Anti-E |
8 |
396 |
|
L252 |
LC |
51 |
M |
Anti-Jka
|
9 |
13 |

Alloantibody specificity varies according to ethnicity. While anti-E and anti-K are the most common alloantibodies in individuals of European descent, anti-K is rare in East Asians, likely because of the low prevalence of K antigen (0–1.4%) in East Asians [
1112] in comparison to individuals of European descent (9%) [
10].
RBC alloimmunization has been reported in 0.5–1.4% of Korean patients in the initial AST [
131415], and one study reported that 0.6% of patients showed positive conversion of AST from an initial negative AST [
13]. In our study, the overall RBC alloimmunization rate was 6.3%, which means that patients with MDS and LC requiring chronic transfusions have higher RBC alloimmunization rates than other transfused patient populations.
In our study, the RBC alloimmunization rate in MDS patients was 4.3%. RBC alloimmunization rates in MDS patients from various Western countries and Australia ranged from 11% to 59% [
81617]. This variation may reflect the differences in patient numbers, study design, treatment, and ethnicity. Few studies have examined RBC alloimmunization in liver disease. Bajpai et al. [
18] reported a 5.2% alloimmunization rate in 842 multitransfused liver disease patients in India, similar to that found in our study for LC patients.
In this study, the cumulative risk of alloimmunization according to the increase in transfused RBC units was significantly higher in LC than in MDS patients. Sanz et al. [
16] reported a 12.4% cumulative risk of alloimmunization after transfusion of 25 RBC units in MDS and chronic myelomonocytic leukemia patients, and the risk plateaued at 19.5% after transfusion of 130 RBC units. Ortiz et al. [
19] reported an 8.3% risk of alloimmunization after transfusion of 25 RBC units in MDS patients and demonstrated a lower rate of alloimmunization in patients treated with azacitidine than in untreated patients, suggesting that immunosuppression by azacitidine leads to the development of immunological tolerance. The immunosuppression effect of the treatment as well as high phenotypic homogeneity of the Korean population are potential causes of the relatively low alloimmunization rate and cumulative risk in the MDS patients in the current study.
In our study, more than half (60%) of the alloimmunized patients developed alloantibodies before transfusion of 10 RBC units. This finding was similar to the finding by Sins et al. [
20] that half of the alloimmunized patients with sickle cell disease developed alloantibodies before transfusion of eight RBC units. Although the number of transfused RBC units is a risk factor for RBC alloimmunization, our results suggest that alloimmunization can develop after a limited number of RBC transfusions in more patients than expected. With respect to the time interval between transfusion and alloantibody detection, alloantibodies were detected within 14 days in three patients (15%) and after 14 days in 17 patients (85%), similar to previously reported rates of 17% and 83% within and after 14 days after transfusion, respectively (
P=0.829) [
1]. Although the direct antiglobulin test was not conducted for three patients with early alloantibody formation within 14 days after transfusion, there was no evidence of hemolytic transfusion reaction in these patients. In two female patients, early alloantibody formation was considered a booster immune response on the basis of their pregnancy history. For a male patient with anti-Jk
a, we could not determine whether early alloimmunization was a primary immune response or a booster response.
We did not investigate the general RBC alloimmunization rate in all patients who received RBC transfusions at Samsung Medical Center during the study period, and further large-scale studies are needed.
In conclusion, Korean MDS and LC patients requiring chronic transfusions had higher RBC alloimmunization rates than other transfused patient populations, and the cumulative risk of alloimmunization according to the increase in transfused RBC units was significantly higher in LC than in MDS patients. Moreover, the developed RBC alloantibodies were from the non-matching of Rh, Duffy, and Kidd systems. Therefore, extended RBC antigen matching of Rh, Duffy, and Kidd blood groups should be a priority for Korean MDS and LC patients requiring chronic transfusions.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download