Malignant melanomas are cutaneous and extracutaneous tumors arising from melanocytes, which are embryological remnants of neural crest cells. Most melanocytes are located in the epidermis and dermis, but are also found in many other locations including the eye, leptomeninges, oral mucous membranes, esophagus, rectum, anal canal, nasal cavity, paranasal sinuses, larynx, vagina, and cervix [
1]. Primary vaginal malignant melanomas are rare and aggressive tumors. Only 500 cases have been reported [
2], principally affecting postmenopausal women, with a mean age of 57 years [
4]. Mucosal melanomas are more often associated with
KIT gene mutations rather than
BRAF mutations, which are more often found in cutaneous melanomas [
5]. Primary malignant vaginal melanoma usually occurs in the anterior wall, typically in the lower one-third. Common clinical presentations are abnormal vaginal bleeding, an easily palpable vaginal mass, pain, and discharge from the vagina [
36]. On visual inspection, these tumors appear as nodular and polypoidal masses that are easily palpable and usually ulcerated, and bleed on touch. They are often pigmented masses, however amelanotic tumors lead to misdiagnosis and delayed treatment [
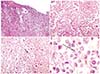
1]. Amelanotic appearance is not rare among mucosal lesions, but makes diagnosis even more difficult. When pigment is absent, even microscopic diagnosis is difficult. Immunohistochemical staining positive for protein S-100, HMB45, Melan-A, Mart-1, and tyrosinase support a diagnosis of melanoma. Therefore, biopsies and immunohistochemical analysis are mandatory in such cases. When diagnosing primary mucosal melanoma, especially in uncommon locations, it is of crucial importance to exclude metastatic lesion from primary cutaneous or ocular melanoma. Whole-body skin examination and ophthalmic examination are crucial. The presence of in situ melanoma/junctional components or a radial growth phase is important for distinguishing primary lesions from metastases or intact epithelium overlaying invasive melanoma. However, because of hidden locations and lack of early symptoms, diagnosis of mucosal melanomas is usually delayed and many lesions are ulcerated at diagnosis; therefore, this criterion is not easy to assess. Hence, the absence of junctional change in an ulcerated lesion does not preclude the possibility of a primary melanoma [
78]. As these cases are uncommon and rarely reported, definitive treatment guidelines are lacking. The Food and Drug Administration (FDA) previously approved interleukin-2 and dacarbazine for distant metastasis; in clinical trials, each had response rates of 10–20% without improving overall survival benefit. Presently, ipilimumab, a fully-humanized antibody that binds to cytotoxic T-lymphocyte-associated antigen 4, has been approved by the FDA for use in metastatic disease [
9].
In conclusion, amelanotic vaginal melanomas are rare tumors and can be easily misdiagnosed on clinical examination as well as on imaging. Therefore, biopsy, histopathological examination, and immunohistochemistry are mandatory for a conclusive diagnosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download