1. Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol. 1983; 7:463–475.
2. Salman MC, Kuzey GM, Dogan NU, Yuce K. Aggressive angiomyxoma of vulva recurring 8 years after initial diagnosis. Arch Gynecol Obstet. 2009; 280:485–487.
3. Chen H, Zhao H, Xie Y, Jin M. Clinicopathological features and differential diagnosis of aggressive angiomyxoma of the female pelvis: 5 case reports and literature review. Medicine (Baltimore). 2017; 96:e6820.
4. Jao SW, Beart RW Jr, Spencer RJ, Reiman HM, Ilstrup DM. Retrorectal tumors: Mayo Clinic experience, 1960–1979. Dis Colon Rectum. 1985; 28:644–652.
5. Messick CA, Hull T, Rosselli G, Kiran RP. Lesions originating within the retrorectal space: a diverse group requiring individualized evaluation and surgery. J Gastrointest Surg. 2013; 17:2143–2152.

6. Siassi RM, Papadopoulos T, Matzel KE. Metastasizing aggressive angiomyxoma. N Engl J Med. 1999; 341:1772.

7. Blandamura S, Cruz J, Faure Vergara L, Machado Puerto I, Ninfo V. Aggressive angiomyxoma: a second case of metastasis with patient's death. Hum Pathol. 2003; 34:1072–1074.

8. Geng J, Cao B, Wang L. Aggressive angiomyxoma: an unusual presentation. Korean J Radiol. 2012; 13:90–93.

9. Han-Geurts IJ, van Geel AN, van Doorn L, den Bakker M, Eggermont AM, Verhoef C. Aggressive angiomyxoma: multimodality treatments can avoid mutilating surgery. Eur J Surg Oncol. 2006; 32:1217–1221.

10. Choi H, Park C, Ji YI. Alternative surgical approaches for aggressive angiomyxoma at different sites in the pelvic cavity. Obstet Gynecol Sci. 2015; 58:525–529.

11. Tyagi V, Dar TI, Durani AM, Chada S. Robotic assisted excision of retrovesical angiomyxoma in a male patient. J Minim Access Surg. 2014; 10:84–86.
12. Patel B, Hussain A.. Aggressive angiomyxoma: a rare presentation. J Case Rep. 2016.

13. Carchman E, Gorgun E. Robotic-assisted resection of presacral sclerosing epithelioid fibrosarcoma. Tech Coloproctol. 2015; 19:177–180.

14. Oh JK, Yang MS, Yoon DH, Rha KH, Kim KN, Yi S, et al. Robotic resection of huge presacral tumors: case series and comparison with an open resection. J Spinal Disord Tech. 2014; 27:E151–E154.
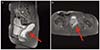
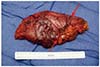
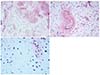




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download