This article has been
cited by other articles in ScienceCentral.
Abstract
We reviewed the anatomical characteristics of the conduction system in the ventricles of human and ungulate hearts and then raised some questions to be answered by clinical and anatomical studies in the future. The ventricular conduction system is a 3-dimensional structure as compared to the 2-dimensional character of the atrial conduction system. The proximal part consisting of the atrioventricular node, the bundle of His and fascicles are groups of conducting cells surrounded by fibrous connective tissue so as to insulate from the underlying myocardium. Their location and morphological characters are well established. The bundle of His is a cord like structure but the left and right fascicles are broad at the proximal and branching at the distal part. The more distal part of fascicles and Purkinje system are linear networks of conducting cells at the immediate subendocardium but the intra-mural network is detected at the inner half of the ventricular wall. The papillary muscle also harbors Purkinje system not in the deeper part. It is hard to recognize histologically in human hearts but conducting cells as well as Purkinje cells are easily recognized in ungulate hearts. Further observation on human and ungulate hearts with myocardial infarct, we could find preserved Purkinje system at the subendocardium in contrast to the damaged system at the deeper myocardium. Further studies are necessary on the anatomical characteristics of this peripheral conduction system so as to correlate the clinical data on hearts with ventricular arrhythmias.
Keywords: Heart conduction system, Purkinje fibers, Tachycardia, ventricular, Myocardial infarction
INTRODUCTION
Anatomical studies on the cardiac conduction system have mainly addressed on the sinus node, atrioventricular (AV) node and His bundle, owing to the technical availability of the morphological assessment of these structure.
1)2)3)4) Historical reviews on the discovery of these components of cardiac conduction system gave us some insights on how scientists discovered the conduction system in human.
5)6)7) The availability of clinical tools to reverse the rhythm disturbance from the atrial chambers have contributed to the further development of anatomical knowledge of the atrial arrhythmia.
8)9) Our understanding on the ventricular conduction system however is still primitive. There was some progress in the clinical evaluation and interventional therapy on the abnormalities of the ventricular conduction system
10)11) but it is still a challenge to understand the 3-dimensional (3D) anatomy of the cardiac conduction system beyond the nodes and bundle of His.
Anatomical techniques to visualize the left/right fascicles and Purkinje system are mostly revivals of classical techniques, being reconstruction of images from serial sections
12) or India ink injection.
4)13) Recently, immunohistochemical techniques have been developed and make it possible to distinguish the myocytes specialized for pacemaking and conduction as opposed to the working myocardium.
14) However, those techniques on the cardiac conduction system in ventricle has not yet been established.
In this review, we summarize our understanding on the morphology of the ventricular conduction system and then raise some questions related to the anatomy and pathology of the ventricular conduction system. Some are answered by speculation or by morphological observation on normal and diseased hearts in human and goats. We have to admit that those morphological studies have many limitations on this complex issue of cardiac rhythm disturbance. We hope clinical electrophysiologists together with anatomists will solve or suggest possible tools to solve the gaps between the bench and the bedside.
THE HISTORICAL SEQUENCE OF THE KNOWLEDGE ON THE CARDIAC CONDUCTION SYSTEM
It is worthwhile to review the historical sequence of discovery of the cardiac conduction system.
5)6) In 1839, Jan Evangelista Purkinje described “the Purkinje system” as a net of “
gelatinous fibers” in the subendocardium of the heart. The second was “the bundle of His” as a conducting bundle between the atrium and the ventricle found by Wilhelm His, Jr in 1893. The third was the “AV node” by Ludwig Aschoff and Sunao Tawara in 1906, which was a “complex node” of tissue at the proximal end of the bundle of His. They concluded that the electrical impulse continued from the AV node through the bundle of His, divided into the bundle branches, and terminated as the Purkinje fibers. Finally in 1907, Arthur Keith and Martin Flack discovered the sinus node, the beginning of the electrical system of the heart.
With the development of arrhythmia ablation procedures, a new field of cardiac pathology has emerged on the anatomical characteristics of the heart and the mechanism of tissue injury in relation to the different subtypes of arrhythmia.
4)5) The first closed-chest ablation of the AV junction was performed in 1982 followed by insertion of the intracardiac pacemaker in patients with drug-refractory supraventricular arrhythmias and the autopsy was performed after 3 years survival.
15) The earliest catheter ablations by radiofrequency energy of accessory AV pathways in Wolff-Parkinson-White syndrome were reported in 1991.
16)
ANATOMICAL PRINCIPLES OF THE VENTRICULAR CONDUCTION SYSTEM
The ventricular conduction system has several different anatomical characteristics compared to the atrial conduction system.
17)18) The myocardial mass of the atrium is relatively thin, and it is more likely dilated rather than hypertrophied in response to the pressure or volume overload. The atrial mass therefore can be considered as a membrane or a 2-dimensional (2D) structure. The ventricular myocardium, in contrast, is thick and the inner and outer parts of the ventricular wall have different characteristics. The inner subendocardial myocardium is more vulnerable to ischemia whereas the outer subepicardial myocardium has less mural tension and more vascular supply from the epicardial coronary arteries. The ventricular wall becomes hypertrophied in response to the pressure and/or volume overload. The ventricular mass therefore has to be understood as a 3D structure. Clinical studies on post-myocardial infarct ventricular tachycardia (VT) showed mostly 2D character such that relatively small anatomically fixed subendocardial tissue is responsible for the arrhythmia. In contrast in hearts with dilated cardiomyopathy and VT the 3D character is evident. Scar related reentry is the most common mechanism and the scar in dilated cardiomyopathy may be mid-myocardial or epicardial and most often occurs in the basal anteroseptal and inferolateral left ventricle (LV) regions.
10)19)
The 3D characteristics of the ventricular myocardium demands a special conduction system within the ventricular mass so that the whole ventricle contracts efficiently to produce systolic contractile power. The Purkinje system is then considered as an intraventricular and 3D conduction network coupling the major conduction bundles to the ventricular myocytes.
Coronary arterial supply to the conduction system is also related to the development of the conduction disturbances. The arterial supply to the AV node arrives from the right coronary artery at the crux cordis which is at the junction between the inferior vena cava to the AV junction. Impairment of the vascular supply to the AV node is inevitable when the right coronary arterial blood supply is impaired. The left main fascicle and Purkinje system are located at the immediate subendocardium of the LV so that direct diffusion can supply oxygen to the conduction tissue even in myocardial ischemia.
The morphology of the fascicles and the Purkinje system may have some basic characteristics of the conduction tissue in the ventricle. For the sake of physiological efficiency of the ventricular contraction the apical part of the ventricle should contract first and then the contraction propagate to the outflow part of the ventricles. The electrical signal has to follow the sequence of apex first. The proximal fascicles and the Purkinje system therefore have to deliver electrical signals far to the apex from the base of the heart (AV node and bundle of His) without contact to the underlying myocytes. Moreover, the conduction tissue should have different contractile function from the underlying myocardium to minimize the friction or sheer force between 2 different systems with different contraction.
The morphological characteristics of the fascicles and the Purkinje system therefore could be drawn through the speculation such that; 1) specialized tissue in bundle or fascicle, 2) insulated from the underlying myocardial tissue, 3) located on the endocardial surface, 4) minimal contractile fibers in the cytoplasm, and 5) side to side conduction among accompanying cells in the tract to synchronize the electrical impulse.
ANATOMY OF THE ATRIOVENTRICULAR NODE AND ATRIOVENTRICULAR JUNCTIONAL STRUCTURE IN HUMAN HEARTS
The AV node, the bundle of His and the left and right bundles are basic proximal conduction system. Considering the importance of the electrical insulation between the atrial and ventricular myocardium, fibrous insulation was recognized as the cardiac skeleton, consisting of annuli of 4 cardiac valves, right and left fibrous trigone and membranous septum (MS). The aortic valve is at the center of 4 valves and the fibrous continuity between the aortic and the mitral valves is the key to the understanding of the cardiac skeleton. The anterior mitral leaflet and the non-coronary cusp of aortic valve are closely approximated and right and left fibrous trigones are formed at each side of the mitral aortic fibrous continuity. The right fibrous trigone is at the center of 3 annuli of 2 AV valves and aortic valve. The MS is also at the vertical plane of the right fibrous trigone. The pulmonary valve annulus is poorly defined and has a separate structure.
The AV node is located at the antero-superior of the coronary sinus, postero-inferior of the MS, on the right atrial wall over the ventricular septum. There is a fibrous insulation between the AV node and the ventricular myocardium. The node consists of compact node and transitional zone between the node and the atrial myocardium (
Figure 1). Histologically the node is a collection of short spindle shaped myocardial cells in contrast to the ribbon shaped myocardial cells at the atrial and ventricular chambers. The nodal cells in the compact zone are tightly attached to each other but the nodal cells in the transitional zone are separated by connective tissue. The atrial myocardium is in close continuity to the AV nodal cells but the ventricular myocardium is free of direct contact to the nodal cells except for the bundle of His.
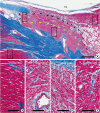 | Figure 1
(A) Low magnification of AV node and neighboring myocardium of atrial and ventricular septa in a human heart (bar=600 μm). Yellow arrows delineate the compact nodal cells and black arrows demarcate transitional zone between the node and atrial myocardium. (B–E) Histology of myocytes of ventricular myocardium (B, 1 in A), compact zone (C, 2 in A), transitional zone (D, 3 in A) of AV node, and atrial muscle (E, 4 in A), respectively (bars of B–E=100 μm).
AV = atrioventricular; RA = right atrium.

|
Basic histology of the AV node is a collection of spindle shaped cells with interstitial connective tissue which contains nerve endings and parasympathetic ganglion cells. There are posterior extensions of nodal cells at the right inferior, left inferior, right superior and left superior aspect of the compact AV node. Superior input to the AV node (the putative “fast pathway”) is located at the anterior portion of the triangle of Koch near the compact node. Inferior input (the putative “slow pathway”) is in the direction of the coronary sinus along the tricuspid annulus. Those 2 pathways facilitate the onset and maintenance of AV nodal reentry tachycardia.
Another type of conduction pathway from the atrial myocardium to the ventricular myocardium is the Kent bundle, which conducts electric impulse through a small number (a few) of myocardial cells. If we examine the heart histologically at the AV junction, it is not rare to find such a close approximation of atrial and ventricular myocardial cells which may be interpreted as a Kent bundle in a morphological sense. Therefore, we understand that a histologically defined Kent bundle is common, but demands some additional change of the micro-environment for functional definition so that the tract is used to conduct electrical impulse to develop ventricular preexcitation.
ANATOMY OF THE BUNDLE OF HIS AND PROXIMAL PART OF THE LEFT/RIGHT BUNDLES
The bundle of His at the original description was a tiny connecting band like channel between the atria and ventricles. The bundle of His is now understood as a cord like collection of spindle shaped conduction cells at the junction between the MS and the ventricular septal crest. The bundle runs at the inferior border of the MS at the crest of the ventricular septum. The exact location of the bundle was on the left side (24/32) or center (3/32) of the ventricular septal crest but it may be on the right side (5/32).
20) Where the bundle arrives at the right fibrous trigone, it is exposed to the left ventricular endocardium and gives rise to the left branch at the left ventricular subendocardium whereas the right branch is in the myocardial layer at the ventricular septum. Both the right and left bundle branches are thin and wide so that they cover proximal part of the summit of the ventricular septum.
Because the left branch is on the left ventricular septal endocardium, we can delineate the width and course of the fascicles. A classical morphological study described variation of the width (1–14 mm) and shape at the proximal end of the fascicle.
20) The shape of the left bundle was more often broad fan shaped “non-division” fascicles (11/13) but some (2/13) had anastomosing narrow stripes (proximal division).
20) Macroscopic morphology of the septal surface is not described yet but our observation showed morphologic variation on the size of trabeculae (
Figure 2). When trabeculae are fine and regular the septal surface will be flat and all the fascicles are on a same septal plane (
Figure 2A). If these trabeculae are very coarse the intervening inter-trabecular spaces are deep such that the fascicles are not in a same septal plane but they are at the crest of these trabeculae (
Figure 2D). We could describe the anterior, middle and posterior rami of the fascicle but in most cases such division is not clear. At the proximal part of the fascicle, the fascicle in fibrous sheath could be lifted up from the underlying myocardial wall. We then speculate the left bundles with proximal division will have thick muscular septal surface. The trabeculae on the apical part of the left ventricular septum are continuation of fascicles or Purkinje system as a continuum of the fascicle.
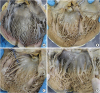 | Figure 2
Morphological variation of the left ventricular septal surface in human hearts as seen from the left after incision of the LV between 2 major papillary muscles and incision at the center of the anterior leaflet of mitral valve. (A) Fine and regular trabeculae throughout the whole wall. (B) Fine trabeculae admixed with a few coarse trabeculae. (C) Coarse and regular medium sized trabeculae. (D) Prominent big trabeculae and deep inter-trabecular spaces.
LV = left ventricle.

|
HISTOLOGY OF BUNDLES, FASCICLES AND PURKINJE SYSTEM
Histology of the left bundle and branches are shown in
Figure 3. The conduction cells at the proximal part of the bundle are similar to the cells in the AV nodes. The cells became more elongated at the distal part of the course of fascicles and some of them have perinuclear vacuoles.
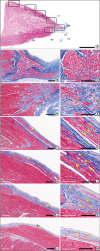 | Figure 3
Histological sections of the ventricular septum reveal the bundle of His, left and right bundles and peripheral branches of the bundle in a human heart. (A) Scanning view of the ventricular septum. Bar represents 5 mm. (B, C) Low and high magnification of the bundle of His (1 in Figure 3A) showing tightly bound small spindle cells in thick fibrous sheath. (D, E) Low and high magnification of the proximal right bundle showing some vacuolated conducting cells in the myocardium (2 in Figure 3A). Conduction cells often show perinuclear clearing (arrows). (F–M) Low and high magnification of distal left bundle and Purkinje fibers (F and G, 3; H and I, 4; J and K, 5; L and M, 6 in Figure 3A). Distal part of the left bundle branches shows bundles of 5–10 cells covered by fibrous sheath. Purkinje cells are peripheral part of the bundle branches showing ground glass appearing cytoplasm rather than fibrillary cytoplasm (bars in B, D, F, H, J, and L represent 400 μm and in C, E, G, I, K, and M represent 200 μm).
LV = left ventricle; MS = membranous septum; RA = right atrium; RV = right ventricle; TV = tricuspid valve.

|
Further tracing of the Purkinje system is hardly successful because of the lack of fibrous sheath and morphological similarity between the myocytes and Purkinje cells at this level. We could only recognize the Purkinje cells by the endocardial location and grouping in a few cells in contrast to the big groups of many myocardial cells in the underlying myocardium. Our morphological understanding on the distal Purkinje system, intramural Purkinje cells deep in the ventricular wall is limited. The Purkinje cells on the papillary muscle is also poorly recognized by histology (
Figure 4).
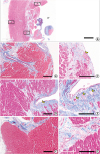 | Figure 4
Histological sections of the apical part of the LV reveals the outer compact layer and the inner trabecular layer of the myocardium in a human heart. This particular case had old myocardial infarct and subendocardial fibrosis is noted. (A) Scanning view of the ventricular wall (bar=5 mm). (B, C) Low and high magnification of one of trabeculae (1 in Figure 4A) showing a few Purkinje cells at the endocardial zone of the trabeculae. (D, E) Other deeper part of the endocardium (“2” in Figure 4A) showing similar Purkinje cells at the endocardium. (F, G) The interstitium deep in the compact myocardium (“3” in Figure 4A) does not show Purkinje cells (bars in B, D and F represent 400 μm and in C, E, and G represent 200 μm).
LV = left ventricle.

|
QUESTIONS ON THE ANATOMY OF THE PURKINJE SYSTEM
We can also raise several questions on the morphology of the ventricular conduction system.
The first is whether the Purkinje system is exclusively in the endocardial surface of the LV or it ramifies to branches within the deeper myocardium of the ventricular wall. If the Purkinje system extends deep to the ventricular wall, we may need higher electrical power to ablate the Purkinje system deep in the myocardial wall.
The second is whether the Purkinje system contributes to the papillary muscle contraction and the location of the Purkinje fibers in the papillary muscle. This question is related to the anatomy of the basal part of the papillary muscle whether they are part of the endocardial Purkinje system or an additional structure over the Purkinje system.
The third is on the vascular supply to the Purkinje system whether they are supplied by the epicardial coronary arteries or by direct diffusion from the endocardium. This will be important issue when there is a myocardial infarct, both transmural or subendocardial.
OBSERVATION ON THE CONDUCTION SYSTEM IN GOAT HEARTS
We recently analyzed the conduction system in hearts of goats and pigs for their location and morphology. This model is particularly useful because the Purkinje system in these animals have special histological features for us to recognize easily.
The gross anatomy of the heart of the sheep and the pig is basically same as that of the human heart although the great vessels have different shape such that they have longer inferior vena cava and arch vessels like a bicornuate aorta. The alignment of major papillary muscles of mitral valve in these animals is not different from human heart. But both the antero-lateral and the postero-medial papillary muscles are leaning to the wall without sub-papillary inter-trabecular spaces. The basal parts of papillary muscles were the part of the ventricular wall in hearts of these animals.
We could find 2 important differences in their conduction system from that in human hearts. The sinus node in human is a subepicardial structure but the sinus node of the pig and the goat was a transmural structure in which there is no atrial myocardium at the endocardial aspect of the atrial wall at the sinus node. The MS of goat and pig hearts is smaller than that in the human heart. The AV part of the MS was not different, but the ventricular part is much smaller in these animals. The MS from the left ventricular aspect is subaortic, at the junction between the non-coronary and left coronary cusps. Because the MS is small the area is almost muscular rather than membranous (
Figures 5 and
6).
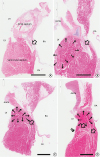 | Figure 5
Scanning view of histological sections of AV node area of a goat heart. (A) The middle of the AV node shows collection of nodal cells (arrow) at the surface of the right atrial wall. (B) Anterior end of the AV node (arrow) is buried underneath the fibrous annulus of the TV near the “MS”. There is a mass of myocardium (delineated with 9 arrows) in a goat heart at the sub-aortic zone of the LV where MS separates LV and the RA in human. (C) More anteriorly the myocardial tissue still present (delineated with arrows) so that the bundle of His (arrow) is still in the myocardium. (D) The bundle of His bifurcates into the right and left bundles (arrows) underneath the myocardial tissue (delineated with arrows) which is the location of the MS in human. The right bundle runs within the myocardium of the septum but the left bundle runs to arrive at the left ventricular surface of the septum (double arrow) so that it is at the subendocardial location like the left bundle in human (bars represent 4 mm).
AV = atrioventricular; LV = left ventricle; MS = membranous septum; RA = right atrium; TV = tricuspid valve.

|
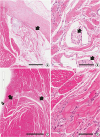 | Figure 6
Low and high magnification of the AV node (A, B) and left and right bundles (C, D) in a goat heart. AV node is a collection of specialized cells and nerve endings (arrows in B). The cells of the left and right bundles (arrows in C) are basically same cells to the node, being characterized by ground glass cytoplasm devoid of myofilaments (D). Double arrow indicates the endocardial location of the left bundle after the intramyocardial course of the proximal part of the bundle in this goat heart.
AV = atrioventricular.

|
The histological study of the conduction system in these animals were carried out in a few hearts. The sinus node and the AV nodes are almost same as those in human hearts. The bundle of His and its continuation to the left bundle is somewhat different in these animals. The bundle of His and left bundle were buried into the myocardium at the subaortic part.
Further morphological studies on the conduction system was carried out in goat hearts with old myocardial infarct. The cardiac lesions in these hearts were produced for previous study.
21) Goat hearts were sectioned for their ventricular conduction system at the infarct and at the preserved area. We could recognize the Purkinje system at the endocardium (
Figure 7A and 7B). The Purkinje system was characterized by their absence of reticulin fibers at the intercellular junction within the bundle. We could also find absence of cytoplasmic myofibrils by Masson's trichrome stain so that the cytoplasm looked empty by hematoxylin-eosin stain. We could further trace the ramified Purkinje cells at the interstitium between myocardial bundles (
Figure 7E–7H).
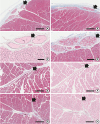 | Figure 7
Purkinje system of the LV in the goat heart. (A, B) Purkinje cells (arrows) are located in the superficial layer of the subendocardium (Masson's trichrome stain). Bars represent 300 μm. (C, D) Magnification of the Purkinje system shows intimate attachment of cells without reticulin fibers (C, Reticulin stain). The cytoplasm free of myofilament (D, Masson's trichrome stain). Bars in C and D represent 100 μm. (E–H) Purkinje cells are also seen at the inter-fascicular interstitium of the myocardium. (E and G, Masson's trichrome stain; F and H, Reticulin stain). Bars represent 300 μm.
LV = left ventricle.

|
The left ventricular wall with myocardial infarct nicely demonstrates extensive loss of myocytes and broad replacement fibrosis (
Figure 8A–8C). The Purkinje system at the endocardium is still alive in these sections. Reticulin stain demonstrate again the Purkinje system.
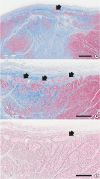 | Figure 8 Purkinje system in goat heart with myocardial infarct. (A) Low magnification of the left ventricular wall shows extensive transmural loss of myocytes and fibrosis in the myocardium. The Purkinje system remains at the endocardial surface of the wall (arrow) (bar=600 μm). Masson's trichrome stain. (B) Magnification of the endocardial part of the section (A) shows details of the Purkinje system (arrows) (bar=300 μm). Masson's trichrome stain. (C) Reticulin staining of the same area of the section (B) shows extensive deposition of reticulin fibers at the zone of myocardial loss. The absence of intercellular reticulin fibers at the Purkinje system is also noted (bar=300 μm).
|
CONCLUSION
The proximal part consisting of the node, the bundle of His and fascicles are groups of conducting cells surrounded by fibrous connective tissue so as to insulate from the underlying myocardium. Their location and morphological characters are well established. The bundle of His is a cord like structure but the left and right fascicles are broad at the proximal and branching at the distal part.
The more distal part of fascicles and Purkinje system are linear networks of conducting cells at the immediate subendocardium but the intra-mural network is detected at the inner half of the ventricular wall. The papillary muscle also harbors Purkinje system not in the deeper part.
Unlike the atria, the ventricular conduction system is a 3D structure. This 3-dimensional characteristic is not always the same, but it is more evident when the VT is associated with dilated cardiomyopathy. In these hearts with myocardial hypertrophy, the ventricles have vast quantities of myocardial tissue. And there is large electrical source that may load mismatches to induce sequential activation with a small number of Purkinje networks. The ventricular conduction system has features to overcome this.
However, retrograde excitation may occur from myocardium to the Purkinje network if aberrant excitation occurs, followed by unidirectional block, which predisposes to re-entry formation.
22) The Purkinje-related ventricular arrhythmias range from isolated ectopies to monomorphic VT or polymorphic VT and ventricular fibrillation, and occur in patients with or without structural heart disease.
It is hard to recognize histologically in human hearts but conducting cells as well as Purkinje cells are easily recognized in ungulate hearts. Further observation on human and ungulate hearts with myocardial infarct, we could find preserved Purkinje system at the subendocardium in contrast to the damaged system at the deeper myocardium.
There are some additional features we observed. The presence of redundant myocardial tissue over the bundle of His in goat hearts may suggest that such abnormal muscle may cause VT in some human victim with abnormal focus near the left ventricular summit. Also, we could observe the morphological variation of trabeculae at the left ventricular septal surface such that the left ventricular fascicles may be on a smooth septal plane in some or at the crest of big trabeculae in some others.
There have been many advances since Tawara's announcement of the Purkinje network in 1906,
12) but so far, many anatomical questions about the Purkinje network have not been resolved. We hope to see more in the future through the combination of basic anatomy and clinical electrophysiology.












 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download