Introduction
Decompressive craniectomy (DC) is performed when there is no response to primary medication therapy in the increased intracranial pressure (IICP) state.
258) However, various complications can occur after DC; one such complication, subdural hygroma (SDG), can be observed in 6% to 21% of patients.
2425) Generally, the SDG that occurs after DC improves naturally.
1) However, 6% to 32% of SDG can be converted to a chronic subdural hematoma (SDH);
4) depending on its degree, surgical treatment such as burr hole trephination may be required.
1)
There are many studies regarding the risk factors of SDG, including injury mechanism (such as motor vehicle accident, fall down injury, blow to head, etc.), Glasgow Coma Scale (GCS) at admission, preoperative midline shifting, accompanying subarachnoid hemorrhage (SAH), compression of basal cistern, and bone flap size of DC. There is no clear consensus in the papers published so far.
1026)
Therefore, the aim of this study was to discover the risk factors and related prognosis of SDG from DC patients.
Materials and Methods
This study is a retrospective review based on a database of patients treated in a single institution from January 2016 to December 2016. A total of 59 patients who underwent DC for IICP management were included in this study. We set the independent variables as follows: 1) patient characteristics including gender, age, history, and etiology, etc.; in addition, patients who received DC due to non-traumatic brain injury (TBI), such as spontaneous cerebral hemorrhage or cerebral infarction were classified in the disease group; 2) preoperative computed tomography (CT) diagnoses such as epidural hemorrhage (EDH), SDH, SAH, intracerebral hemorrhage (ICH), intraventricular hemorrhage (IVH), without distinction between trauma and non-trauma; 3) preoperative CT finding such as basal cistern compression, cortical opening. And the cortical opening is defined as the SAH of the cortex and the ICH are in the same region; 4) operation parameters such as hemilaminectomy, duroplasty, bone flap size (
Figure 1), distance of craniectomy margin from midline, extraventricular drainage; 5) prognostic value such as final Glasgow Outcome Scale (GOS) and length of hospital stay (HD). The dependent variables of this study are the event of postoperative SDG (
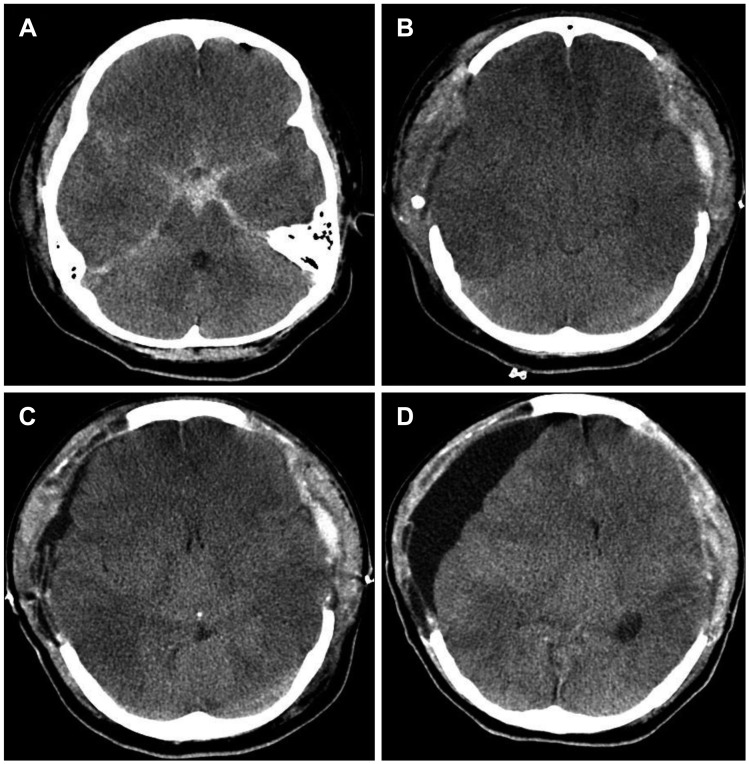
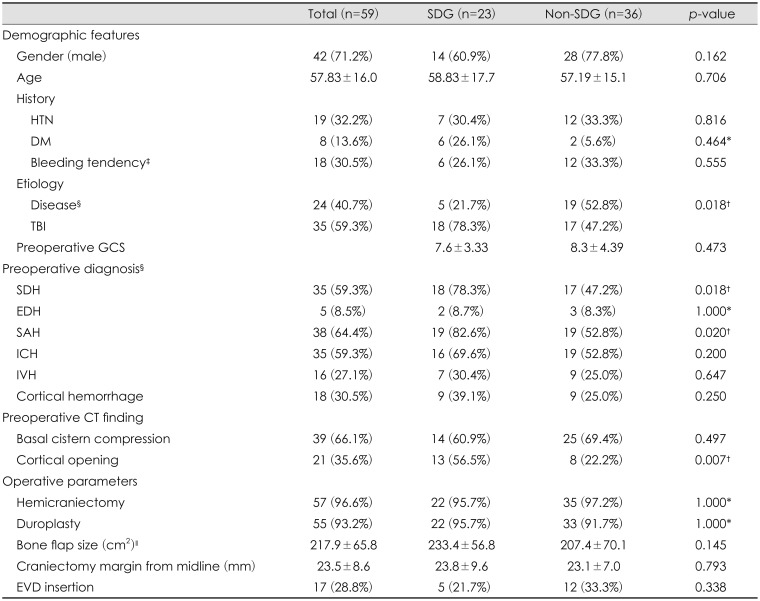
Table 1). SDG was diagnosed if there was fluid collection in the subdural space on the CT scan from the immediate postoperative day to the last follow up day, even if there was no neurological change or mass effect.
Data are expressed according to the properties of the variable. Continuous variables are presented as mean and standard deviation. Categorical variables are presented as frequency and percentage. In order to compare the two groups, we performed the two-sample t-test or χ2 test (Fisher's exact test) as appropriate. Logistic regression analysis was used to identify the factors to predict SDG and the results were expressed as odds ratio (OR) with 95% confidence interval (CI). A p-value less than 0.05 was considered statistically significant and all statistical analyses were conducted using SPSS version 24 (IBM Corp., Armonk, NY, USA).
Results
The overall SDG rate after DC was 39% (23 patients) (
Figure 2). There were 42 males and 17 females who underwent DC. The mean age of the patients was 57.8 years. The patients' age and gender were not significantly different between the SDG group and the non-SDG group. There was also no significant difference between the SDG group and the non-SDG group in terms of the patient's past history such as hypertension, diabetes mellitus, and bleeding tendency due to liver disease, using antiplatelet agent, and preoperative coagulopathy. Moreover, preoperative GCS showed no significant difference between SDG and non-SDG groups. In studying the etiology of brain injury, the incidence of SDG was higher in patients who underwent DC for TBI than in patients who received DC for disease (cerebral infarction, spontaneous brain hemorrhage, etc.) (
p=0.018). Preoperative diagnosis showed a statistically significant higher incidence of postoperative SDG in patients with SDH (
p=0.018) and SAH (
p=0.020). Preoperative CT findings showed a statistically significant higher incidence of postoperative SDG in patients with cortical opening (
p=0.007). However, there was no significant difference between the SDG and non-SDG groups in the operative parameters (
Table 1).
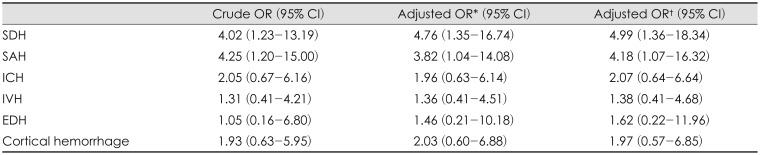
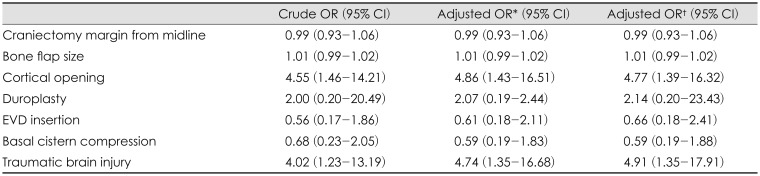
Under logistic regression between the preoperative diagnosis and the occurrence of SDG, there was a statistical association with SDH (OR, 4.99; 95% CI, 1.36–18.34) and SAH (OR, 4.18; 95% CI, 1.07–16.32). The other diagnoses such as EDH, ICH, IVH, and cortical hemorrhage were not related to occurrence of SDG after DC (
Table 2).
Also, under logistic regression between the pre/postoperative risk factors and the occurrence of SDG, there was a statistical association with preoperative cortical opening (OR, 4.77; 95% CI, 1.39–16.32) and preoperative TBI (OR, 4.91; 95% CI, 1.35–17.91). The other pre/postoperative risk factors were not related to occurrence of SDG after DC. That is, the incidence of SDG was higher when DC was performed with TBI or when there was an accompanying preoperative cortical opening (
Table 3).
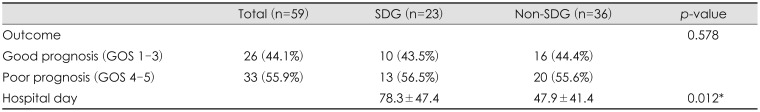
The GOS was compared between the SDG and non-SDG groups. Good prognosis (GOS 1–3) was observed in 10 patients (SDG) and 16 patients (non-SDG), and poor prognosis (GOS 4–5) in 13 patients (SDG) and 20 patients (non-SDG). There was no statistically significant difference in GOS score between the two groups (
p=0.578). However, HD was significantly higher in the SDG group than in the non-SDG group (78.3 days vs. 47.9 days) (
p=0.012) (
Table 4).
Discussion
DC is performed when there is no response to primary medication therapy in the IICP state.
258) Several complications have been reported after DC for IICP management. Postoperative hemorrhage, brain herniation through craniectomy defect, post traumatic epilepsy, intracranial infection, cerebrospinal fluid (CSF) leak, hydrocephalus (HDC), trephined syndrome, SDG, etc. may occur.
25)
Among these possible complications, SDG, which was first reported by Mayo in 1894, is defined as the accumulation of CSF in the subdural space due to rupture of the arachnoid membrane after headinjury.
15) The incidence of SDG has been reported to vary from 4% to 15% in patients without DC and from 6% to 21% in patients who underwent DC.
2425)
There are several theories for the development of SDG, including traumatic shearing stress. In electron microscopy studies by Hanies et al.
6) and Schachenmayr and Friede
19), there is no dead space between the dura and arachnoid layer. The dural border cell layer is vulnerable to physical stress due to the fewer collagen fibers and grabbing cellular elements through amorphous materials, compared with periosteal and meningeal dura, which have many collagen fibers that hold cellular structure.
719) Although the subarachnoid space is protected by a desmosome, a tight junction, and a gap junction that form the trabeculae of the arachnoid barrier layer, this structure is susceptible to damage by shearing stress.
619) The damaged arachnoid membrane produces a CSF flow with a one-way valve, and this ICP gradient between the cerebral hemispheres results in SDG.
18)
The second theory is the occurrence of SDG by iatrogenic surgical disruption. Along with DC, most of the patients underwent duroplasty, resulting in the extension of the arachnoid-dura layer. Aarabi et al.
1) reported that more than 90% of SDG occurred after DC, which occurred in the craniectomy site; however, the incidence of SDG in brain injury patients without DC showed different results. Koizumi et al.
11) reported that SDG showed a similar ratio on both sides; St John and Dila
20) reported that 32 of the 63 cases reported were bilateral, 12 cases were on the right, and 19 cases were on the left. Considering these reports, it can be deduced that iatrogenic disruption will affect the occurrence of SDG. Moreover, there are other hypotheses involving arachnoid rupture, arachnoid flap, blood brain barrier failure, and brain atrophy.
1420)
Generally, SDG occurrence peaks at 3 to 4 weeks after DC, and spontaneously decreases at 14 to 17 weeks.
1) However, in some cases, neurological symptoms are worsened due to an increase in SDG size, and surgical intervention may be necessary.
9)
Additionally, SDG can be converted to chronic SDH; most SDGs exhibit xantho-chromic, containing high red blood cells and proteins,
312131617212223) as a result, inflammation of the subdural space, neo-membrane formation, and fragile and leaky capillaries were made.
41617) This maturation of the SDG membrane and the fragile and leaky capillaries around the stretched parasagittal sinus are vulnerable to internal bleeding, resulting in the conversion of benign SDG to chronic SDH.
41215) Approximately 6% to 32% of SDG changes to chronic SDH;
4) if the size increases and midline shifting progresses, surgical treatment may be required.
1)
For this reason, many studies have been conducted on the risk factors of SDG. Midline shifting (>5 mm), accompanied by HDC, basal cistern compression, arachnoid tearing, and SAH are among the risk factors of SDG reported in various studies.
1026)
Jeon et al.
10) reported that midline shifting (>5 mm), SAH, delayed HDC, basal cistern compression, and arachnoid membrane tearing were risk factors for SDG after DC. However, according to a study by Yuan et al.
26), the risk factors for each of the patients with SDG without DC, those with DC on one side, those with DC on the opposite side, and the patients with DC on both sides, were different. In the case of unilateral DC, temporal hematoma, contusion and traumatic SAH on the non-DC side were related to the incidence of SDG on the operated side. Also, frontal hematoma, frontal contusion, and SDH in the contralateral side of DC increased the risk of SDG on the operated side. And hemorrhage of basal cistern increased SDG incidence in the DC group. The results indicate that the risk factors are different for those cases in which DC is not performed, unilateral DC or bilateral DC are performed. They also argue that arachnoid tearing is a risk factor for SDG.
26)
In our study, the incidence of SDG after DC was statistically significantly associated with preoperative SDH or SAH, and patients with TBI compared with the disease group. Those patients with cortical opening in preoperative CT had a statistically significant association with the incidence of SDG after DC. In addition, although the occurrence of SDG affects the patients' length of hospital stay, it does not have a significant effect on the prognosis of the patient.
Although SDG occurrence is not related to the prognosis of the patients who underwent DC, the complication development, such as chronic SDH, may be related. Therefore, it is necessary to further study a larger sample size for the risk factors of SDG.
This study is limited to a small number of patients in a single institution, and it is difficult to know the results in long term follow up. Moreover, there is a limitation in collecting the data retrospectively.
However, unlike other studies, we did not only investigate the risk factors of SDG in TBI patients but also investigated the risk factors for the occurrence of SDG in all patients who underwent DC due to IICP. Our study has significant implications for the overall prognosis of patients considering the length of hospital stay.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download