Abstract
Objective
Post-traumatic hydrocephalus (PTH) is a frequent and serious complication following brain injury. The incidence of PTH varies greatly among studies. The purpose of this study was to investigate the incidence and treatment of PTH in patients with head trauma.
Methods
We examined 956 patients with head trauma who visited our center from January 2012 to December 2015. The hydrocephalus diagnosis was based on radiologic findings and clinical features, and patients were classified into the mild (Group 1, Glasgow Coma Scale score [GCS] 13–15), moderate (Group 2, GCS 9–12), or severe (Group 3, GCS 3–8) brain injury group according to their GCS at admission. To compare these groups, we used age, gender, radiologic findings, PTH developmental period, and postoperative results (Glasgow Outcome Scale).
Results
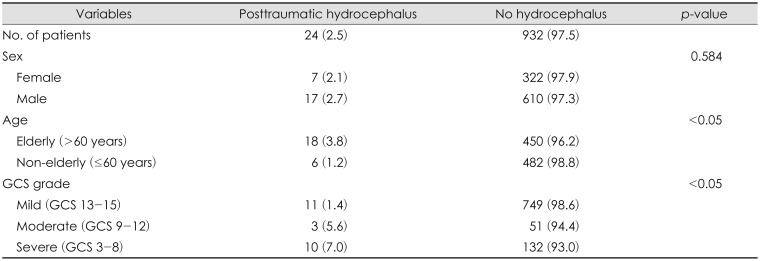
Of the 956 patients, 24 (2.5%) developed PTH. PTH occurred in 11 (1.4%), 3 (5.6%), and 10 (7.0%) patients in Groups 1, 2, and 3, respectively. Of the 24 patients with PTH, 22 (91.7%) developed PTH within 12 weeks post-trauma; the higher the GCS, the later the onset, and the lower the GCS, the earlier the onset (p=0.019). Twenty-one patients underwent ventriculoperitoneal shunting, and 13 had improved symptoms.
Conclusion
The incidence of PTH cannot be ignored. The possibility of PTH needs to be considered in patients with head trauma and appropriate follow-up should be undertaken. PTH is a treatable complication and patients' quality of life and neurological status can be improved if the appropriate treatment is selected and applied.
Posttraumatic hydrocephalus (PTH) was first reported in 1914.18) The incidence of PTH has been reported to be as low as 0.7% to 1.5%, and as high as 29%.11) However, using the computed tomography (CT) criteria for ventriculomegaly, the incidence has been reported to be 30% to 86%. PTH is a progressive process in which excessive cerebrospinal fluid (CSF) accumulates due to liquorodynamic disturbances following brain injury.17) Such brain injuries include acute and chronic subdural hematoma (SDH), epidural hemorrhage (EDH), diffuse axonal injury (DAI), traumatic subarachnoid hemorrhage (SAH), and cerebral contusion.
A wide variety of clinical and radiological diagnostic criteria have been suggested for PTH.3) Previously, only patients with symptomatic hydrocephalus underwent neuroradiological evaluation.9) Therefore, recognition of PTH is often confounded by attributing unresolved or additional symptoms to primary or secondary injury inflicted upon the brain by the trauma.17) However, CT or magnetic resonance imaging (MRI) can be useful aiding tools for hydrocephalus by evaluating the size of the frontal horn, temporal horn, and third ventricle, as well as the periventricular density and modified frontal horn index (mFHI).20)
In general, PTH is associated with severe brain injury, but also has a significant incidence rate in mild brain injury. Therefore, it is necessary to study the clinical features and outcome of PTH after brain injury.
We conducted a retrospective study including 956 patients from author's center who experienced mild to severe brain injury between January 2012 and December 2015. The diagnosis of hydrocephalus was based on the radiologic examination and the clinical condition of the patient during acute period and/or rehabilitation. Radiologic examination was performed from the beginning of the trauma, and was performed whenever necessary for purposes such as the degree of severity, neurological status, changes in clinical status, and follow-up. There is high incidence of ventriculomegaly after trauma. And it is very important to distinguish it from hydrocephalus, but there have not been way to have definite accuracy. In the present study, the diagnosis of hydrocephalus was based on the patient's radiological and neurological changes, such as the enlargement of the ventricle, the presence of interstitial edema or absence of sulci dilation, and the associated symptoms or clinical change in period of ventricle expansion. Based on these, we distinguished it from atrophy and simple ventriculomegaly.
For all patients, we assessed Glasgow Coma Scale (GCS) score at admission, age, sex, diagnosis (based on CT and MRI findings), time to development of PTH, clinical features of PTH, and Glasgow Outcome Scale (GOS) score at the time of diagnosis of PTH and GOS after ventriculo-peritoneal shunt (VPS). Patients were divided into two groups according to their age: an elderly group (≥61 years) and a non-elderly group (≤60 years). Diagnoses were made with CT and MRI at admission, and included all cerebral hemorrhage of acute to chronic phase. We assessed the GCS at admission and subsequently divided all patients into three groups according to their scores: a) Group I, mild brain injury, GCS 13–15; b) Group II, moderate brain injury, GCS 9–12; c) Group III, severe brain injury, GCS 3 to 8. We evaluated time to development of PTH from the date of admission to the day of PTH symptom onset. Time to development of PTH was divided into four durations: within 1 week, 1 to 4 weeks, 4 to 12 weeks, and after 12 weeks.
We evaluated clinical features at the time of PTH diagnosis, and also performed a lumbar tap test in patients who developed PTH. The lumbar tap test was conducted under sterile conditions with a 21-gauge needle. On average, 50 cc of the CSF was drained, and patients underwent absolute stabilization after the procedure.
VPS procedures were performed in patients who showed improvement of symptoms after the lumbar tap test. Although radiologic diagnosed with ventriculomegaly, the surgical indication was performed only when the representative hydrocephalus symptoms dramatically improved with a lumbar test. We conducted GOS at the time of diagnosis of PTH and GOS about one-week after VPS. GOS scores were used to categorize patients as follows: good recovery, moderate disability, severe disability, persistent vegetative state, and death.6)
In the image findings, 64 patients with ventriculomegaly were diagnosed, and 24 patients with symptomatic improvement after lumbar tap test were defined PTH group. The mean mFHI of PTH group was 0.37. Of 24 patients with PTH, 17 (70.8%) were male and 7 (29.2%) were female, but no significant relationship was observed between sex and PTH (p=0.584). Of the 956 patients, 468 (49.0%) were categorized into the elderly group and the remaining 488 (51.0%) were classified as non-elderly, with an overall mean age of 56.7 years. There was a higher incidence of PTH in the elderly group (n=18; 3.8%) than in the non-elderly group (n=6; 1.2%), and the difference was statically significant (p<0.05). There was a significantly higher incidence of PTH in the severe brain injury group (Group I: 11/760 patients, 1.4%; Group II: 3/54 patients, 5.6%; 10/142 patients, 7.0%; p<0.05; Table 1). Of 24 PTH patients, 21 received VPS surgery, excluding three due to refusal of surgery.
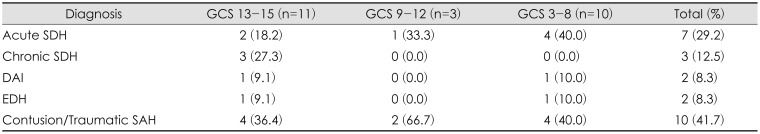
CT and MRI at admission revealed a range of findings from acute to chronic hemorrhage, with some patients having a combined type of hemorrhage. In most trauma, traumatic SAH and contusion are mixed and classified into one category. The main diagnosis was cerebral contusion and/or traumatic SAH in 10 patients (41.7%). The rest of results were as follows: acute SDH in 7 (29.2%), chronic SDH in 3 (12.5%), EDH in 2 (8.3%), and DAI in 2 (8.3%).(Table 2).
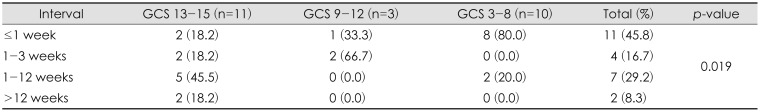
The interval between trauma and development of PTH was variable, with 91.7% of patients (n=22) developing PTH within 12 weeks. Patients with higher GCS scores showed later onset of PTH, while those with lower GCS scores showed early onset. This difference was statistically significant (p=0.019; Table 3). It is thought that the low GCS score indicates a severe brain injury condition when compared to the high GCS, which is caused by relatively low brain compliance, high intracranial pressure (ICP), and rapidly increased outflow resistance of CSF.
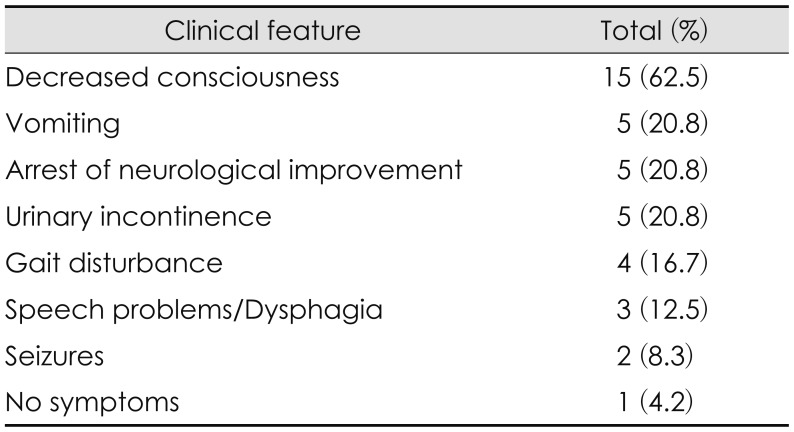
Clinical features of PTH were varied. Among the 24 patients with PTH, we observed symptoms such as decreased consciousness, vomiting, arrest of neurological improvement, urinary incontinence, gait disturbance, speech problems, and first seizures after PTH diagnosis. The majority of patients had complex symptoms, and the most common symptom was decreased consciousness (Table 4).
Symptomatic improvement after lumbar tap test was observed in 24 patients. We recommended VPS surgery for them, but three patients refused and performed a VPS in all of the rest.
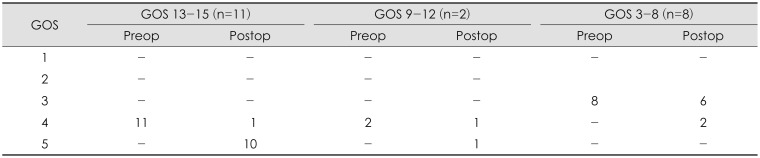
Of those receiving VPS surgery, 13 (61.9%) showed a subsequent improvement in their GOS score immediately. In Group I, 11 patients (52.4%) with PTH received a VPS, with 10 (90.9%) showing subsequent improvement from preoperative GOS 4 to GOS 5. In Group II, 2 patients (9.5%) received a VPS, with 1 (50.0%) showing subsequent improvement from GOS 4 to GOS 5. In Group III, 8 patients (38.1%) received VPS surgery, with 2 (25.0%) showing subsequent improvement from GOS 3 to GOS 4, and 6 (75.0%) showing no improvement (Table 5).
Delayed development of ventricular enlargement following head injury is a well-recognized clinical manifestation.10) Mazzini et al.13) suggested that ventricular enlargement is a common finding in the post-acute phase of severe traumatic brain injury, with an incidence between 30% to 86%. Nonetheless, the presence of ventriculomegaly does not necessarily result in PTH. Indeed, Kishore et al.9) observed that only 13.7% of patients with ventriculomegaly had PTH. Also, in comparison with ventriculomegaly, the exact incidence of PTH is not well known.18) Possible causes of ventriculomegaly include an atrophic process secondary to DAI, a secondary CSF absorptive deficit, or a combination of both these phenomena, as well as obstructed CSF flow with associated increased CSF pulsatility. Furthermore, the repeated application to the brain of small forces that lead to the gradual destruction of periventricular white matter axons via hypoxic, ischemic, and mechanical stress mechanisms can lead to ventricular enlargement.14) To find predictors of increased ventricular size, several authors have studied the possible relationship between posttraumatic ventriculomegaly and various clinical or radiological parameters, with conflicting results.
Owing to the broad spectrum of presentations, from asymptomatic ventriculomegaly to symptomatic PTH, it is challenging to distinguish PTH from compensatory ventricular enlargement after brain parenchymal damage. Affected patients can have varied symptoms, including obtundation, fluctuating consciousness levels, motor deficits, and emotional disturbances.17) Furthermore, there is a recognized normal pressure hydrocephalus (NPH) like syndrome in a subset of patients following head injury. Kawkami et al.8) proposed an incidence of posttraumatic NPH as between 3.7% and 7.5% among their patients.
Information about PTH resulting from head injury is limited compared to the information about these alternative explanations for patients reaching a neurological plateau or showing deterioration. Following head injury, patients may often show slow improvement in terms of consciousness state, stop improving, and then show worsening in their condition. Subsequently, they again become drowsy, lethargic, or comatose, with evidence of increasing ICP. Besides, PTH is a serious complication that may occur in the short-term or long-term after traumatic brain injury. So, with these symptoms, PTH develops in the post-acute phase of head injury, weeks or months later. Also, cognitive changes in patients with head injuries are often multifactorial. For this reason, failure to identify hydrocephalus is not uncommon and this is especially true when the surgeon has adopted a fatalistic acceptance of an unfortunate outcome.2) Therefore, it is important to differentiate neurological deficits secondary to the primary brain parenchymal injury from deficits associated with PTH.18)
The appropriate management of ventricular dilation following brain injury remains controversial because it has been difficult to determine whether ventriculomegaly is related to an atrophic process or due to true hydrocephalus with a CSF absorptive deficit. Therefore, it can be helpful to evaluate patients with CT to further guide management because CT radiologic criteria have been proposed for the diagnosis of hydrocephalus. Among these criteria, the FHI and Evan index are the most popular.6) Nonetheless, conventional methods of calculated FHI often underestimate the index when there is widening of the subdural space. For this reason, we used the mFHI in this study.
Several papers investigating PTH have found various contributing clinical factors. Age, presence of SAH, duration of intensive care unit hospitalization, and decompressive craniectomy (DC) have been identified as risk factors predisposing to PTH. Albeck et al.1) suggest that the efficiency of CSF reabsorption decreases with age. Consistently, another study found significant associations between increasing age and PTH.119) Similarly, our results support a relationship between age and the development of PTH. It is possible that there is a greater extent of meningeal fibrosis in the older population, which causes impairment of CSF circulation and reduced CSF absorption. Age-related cerebral atrophy is also thought to allow accumulation of larger amounts of traumatic intracranial blood, which contributes to CSF circulatory disturbance.10)
Although studies of PTH have reported on the condition in patients with severe brain injury, there have been few studies of the occurrence of PTH in patients with mild brain injury. Poca et al.16) have reported on PTH and outcome in patients with moderate to severe brain injury. Our study therefore extends previous findings by including patients with mild brain injury. We observed a statistically significant difference in the incidence of PTH depending on GCS score at the time of admission. The incidence of PTH in patients with mild brain injury was not statistically significant, but it is important to note that there were patients who required treatment due to the development of PTH.
Patients with severe brain injury had a high incidence of PTH, which may have been associated with surgical treatment such as DC. In particular, DC has been found to be associated with development of PTH, as it can alter CSF pressure dynamics, causing mechanical blockage around convexities or inflammation of arachnoid granulations due to post-surgical debris. This can lead to flattening of normally dicrotic ICP waveforms due to transmission of a pressure pulse through the open cranium. Since arachnoid granulations function as pressure-dependent one-way valves from subarachnoid space to draining venous sinuses, the disruption of pulsatile ICP dynamics results in decreased CSF outflow. 1721)
The time required for the development of PTH has not been established, but studies have suggested that symptoms may begin immediately after trauma.4) The need for shunting may not only occur in the acute stage of injury during which access to neurosurgical intervention and imaging is readily available. Delayed changes in CSF dynamics may cause PTH to emerge weeks or months after onset of injury and long into the rehabilitation phase.7) According to the study, the difference in the time of occurrence varied. Ojemann et al.15) reported that 3 weeks may be sufficient for the development of PTH and Marmarou et al.11) reported that hydrocephalus occurred within 2 weeks after trauma in most patients, and within 1 month in all patients. In our study, 45.4% of patients with minor brain injuries developed PTH within 4 to 12 weeks, which was delayed compared with patients with severe brain injury. This study is a retrospective study that depends on medical record, so onset of PTH may be delayed than previous study due to unexpected conditions such as transfer to rehabilitation department.
In previous studies, SAH has been cited as the most important pathology leading to development of PTH.19) In contrast, Poca et al.16) reported that there was no relationship between PTH and the presence of subarachnoid hemorrhage or type of lesion. Also, we no found a statistically significant association between PTH and SAH. In our study, acute and chronic SDH were more common in patients with PTH than in SAH, and chronic SDH was more common in patients with mild brain injury. Unlike other reports suggesting an association of SAH with PTH, the high rates of acute and chronic SDH were due to the primary diagnosis of major bleeding on CT, which meant that coexisting SAH was overlooked.
Two mechanisms may underlie the etiology of PTH. The first results from increased numbers of cells or proteins in the CSF (from hemorrhage), which causes clogging of CSF outflow and leads to obstruction, as there is reduced absorption via the arachnoid granulations. This results in decreased conductance and increased resistance to CSF outflow. This mechanism can explain that low GCS scores is associated with severe brain injury as compared to high GCS scores, leading to a rapid development of PTH as brain compliance decreases, ICP increases, and CSF outflow resistance increases.
The second mechanism is due to leptomeningeal fibrosis, particularly in the sulci of the convexities, and arachnoid adhesions causing scarring in the basal cisterns and leading to disturbances in CSF dynamics. There is an initial increase in CSF pressure that results in enlargement of the ventricles. Other prominent findings in PTH include ependymal destruction and the presence of subependymal gliosis, together with loss of white matter, particularly around the ventricles.24522) Autopsy studies reveal that transtentorial herniation of sufficient severity to plug the tentorial opening with the brain stem and the herniated part of the temporal lobe will distort and compress the aqueduct to such an extent that the canal may be obstructed or stenosed. In accordance with Laplace's law, ventricular enlargement is maintained even after normalization of pressure after chronic SDH.522)
Shunt surgery for PTH is a widely performed treatment that has been shown to benefit from several papers.1113) At this time, several tests have been proposed to identify “shunt responders” and reduce risk, including a cisternogram, lumbar tap test, external lumbar drainage, measurement of CSF resistance, and long-term recording of ICP.12) However, The treatment of PTH in patients who undergo DC is a difficult problem to solve. Shunt operation with or without cranioplasty may be done simultaneously with aims to correct the underlying problems. The former may result in neurological deficits known as ‘the syndrome of the sinking skin flap’ or ‘the symptom of the trephine’ due to midline shifting and CSF over-drainage at atmospheric pressure.19) The latter procedure is associated with a higher risk of developing brain edema and infection due to early operation. The sequence of treatment should be chosen with regard to the various clinical situations.
This study was conducted as a retrospective study, depending on the medical records. Therefore, an unexpected factor may cause errors such as the fact that the patient has a PTH but the diagnosis is not made or the diagnosis time is delayed. And, the number of patients enrolled in this study is not large, and it is difficult to generalize the conclusion. We tried to differentiate PTH from atrophy and simple ventriculomegaly considering various situations, but there is no accurate method yet. So, clear diagnostic criteria should be studied to distinguish between ventriculomegaly and hydrocephalus.
In our study, the incidence of PTH cannot be ignored, and especially, the incidence rate was higher in patients with older or severe brain injury. Considering this clinical situation, the possibility of PTH is considered and appropriate follow-up is needed in the patients with head trauma. Although the incidence of PTH is lower than that of patients with severe brain injury, it may also occur in patients with mild brain injury. Therefore, careful follow-up is also necessary in mild head trauma. PTH is a treatable complication. So we can improve the quality of life of patients and help to restore their neurological status if the treatment group is appropriately set and treated.
References
1. Albeck MJ, Skak C, Nielsen PR, Olsen KS, Børgesen SE, Gjerris F. Age dependency of resistance to cerebrospinal fluid outflow. J Neurosurg. 1998; 89:275–278. PMID: 9688123.

3. Choi I, Park HK, Chang JC, Cho SJ, Choi SK, Byun BJ. Clinical factors for the development of posttraumatic hydrocephalus after decompressive craniectomy. J Korean Neurosurg Soc. 2008; 43:227–231. PMID: 19096601.

4. Daou B, Klinge P, Tjoumakaris S, Rosenwasser RH, Jabbour P. Revisiting secondary normal pressure hydrocephalus: does it exist? A review. Neurosurg Focus. 2016; 41:E6.

5. Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965; 2:307–327. PMID: 5889177.
6. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1:480–484. PMID: 46957.
7. Kammersgaard LP, Linnemann M, Tibæk M. Hydrocephalus following severe traumatic brain injury in adults. Incidence, timing, and clinical predictors during rehabilitation. NeuroRehabilitation. 2013; 33:473–480. PMID: 23949078.

8. Kawakami K, Yoshino H, Igarashi H, Chiba Y, Yoshino G, Hirose T. A case of idiopathic normal pressure hydrocephalus in an elder diabetic patient. Nihon Ronen Igakkai Zasshi. 2017; 54:186–190. PMID: 28592739.

9. Kishore PR, Lipper MH, Miller JD, Girevendulis AK, Becker DP, Vines FS. Post-traumatic hydrocephalus in patients with severe head injury. Neuroradiology. 1978; 16:261–265. PMID: 740188.

10. Low CY, Low YY, Lee KK, Chan SP, Ang BT. Post-traumatic hydrocephalus after ventricular shunt placement in a Singaporean neurosurgical unit. J Clin Neurosci. 2013; 20:867–872. PMID: 23415060.

11. Marmarou A, Foda MA, Bandoh K, Yoshihara M, Yamamoto T, Tsuji O, et al. Posttraumatic ventriculomegaly: hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J Neurosurg. 1996; 85:1026–1035. PMID: 8929491.

12. Marmarou A, Young HF, Aygok GA, Sawauchi S, Tsuji O, Yamamoto T, et al. Diagnosis and management of idiopathic normalpressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005; 102:987–997. PMID: 16028756.

13. Mazzini L, Campini R, Angelino E, Rognone F, Pastore I, Oliveri G. Posttraumatic hydrocephalus: a clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome. Arch Phys Med Rehabil. 2003; 84:1637–1641. PMID: 14639563.
14. McAllister JP 2nd, Williams MA, Walker ML, Kestle JR, Relkin NR, Anderson AM, et al. An update on research priorities in hydrocephalus: overview of the third National Institutes of Healthsponsored symposium “Opportunities for Hydrocephalus Research: Pathways to Better Outcomes”. J Neurosurg. 2015; 123:1427–1438. PMID: 26090833.

15. Ojemann RG, Fisher CM, Adams RD, Sweet WH, New PF. Further experience with the syndrome of “normal” pressure hydrocephalus. J Neurosurg. 1969; 31:279–294. PMID: 5811831.

16. Poca MA, Sahuquillo J, Mataro M, Benejam B, Arikan F, Báguena M. Ventricular enlargement after moderate or severe head injury: a frequent and neglected problem. J Neurotrauma. 2005; 22:1303–1310. PMID: 16305318.

17. Sarkari A, Gupta DK, Sinha S, Kale SS, Mahapatra AK. Post-traumatic hydrocephalus: Presentation, management and outcome - An apex trauma centre experience. Indian J Neurotrauma. 2010; 7:135–138.

18. Sheffler LR, Ito VY, Philip PA, Sahgal V. Shunting in chronic posttraumatic hydrocephalus: demonstration of neurophysiologic improvement. Arch Phys Med Rehabil. 1994; 75:338–341. PMID: 8129589.

19. Tian HL, Xu T, Hu J, Cui YH, Chen H, Zhou LF. Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg Neurol. 2008; 69:241–246. PMID: 17707493.

20. Tsuang FY, Huang AP, Tsai YH, Chen JY, Lee JE, Tu YK, et al. Treatment of patients with traumatic subdural effusion and concomitant hydrocephalus. J Neurosurg. 2012; 116:558–565. PMID: 22175725.

21. Waziri A, Fusco D, Mayer SA, McKhann GM 2nd, Connolly ES Jr. Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery. 2007; 61:489–493. PMID: 17881960.

22. Yoshimine T, Hayakawa T, Kamikawa K, Ohnishi T, Yamamoto K. Partial hydrocephalus with chronic subdural hematoma. Neurosurgery. 1982; 11:698–702. PMID: 6984144.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download