Abstract
Objective
Rapid expansion of subacute subdural hematomas (saSDHs) is an uncommon complication in the course of acute subdural hematomas (SDHs). The current study evaluated relevant factors and treatment methods for saSDHs with neurologic deterioration and mass effect.
Methods
A saSDHs was chronologically defined as an SDH occurring 4 to 21 days after head trauma. All cases of surgically treated SDHs were retrieved from the head trauma bank at our institution. Twenty-three patients with expanding saSDHs who met the following criteria were enrolled in the study: defined age of the hematoma, clinical deterioration, and radiological expansion of the hematoma. Cases were analyzed according to demographic factors, trauma mechanism, medical co-morbidity, and surgical method.
Results
Expanding saSDHs occurred more often in older (≥60 years old) than in younger patients (69.6% vs. 30.4%, respectively); they also occurred more often in men than in women (64% vs. 36%, respectively). Antiplatelet or anticoagulant therapy was used in 52% of patients. The Glasgow Coma Scale score was 13 at the time of the trauma and deteriorated to 11 at the time of surgery. The mean time from the trauma to development of the expanding saSDH from an SDH was 13.3 days. Regarding surgical methods, closed-system drainage was performed in 22 patients, and only one patient underwent craniotomy with hematoma removal. All patients exhibited neurological improvements after surgery.
Go to : 
Subdural hematomas (SDHs) can be divided into three groups based on chronology: acute, subacute, and chronic. A subacute SDH (saSDH) is defined as a hematoma that evolves from an acute SDH within 4 to 21 days of head injury.15) A chronic SDH, which occurs 21 days after head injury, is one of the most common traumatic conditions in the elderly population and does not necessarily develop from an acute SDH.911) However, a problematic saSDH is a rare complication in the usual course of an acute SDH.581618)
Expanding saSDHs can be defined as initially non-operated acute SDHs that show rapid neurological deterioration and expansion of hematoma volume in the subacute stage. Surgical intervention should be applied for the expanding saSDH and should not be overlooked. However, no definite surgical indication has been established for saSDHs. Several factors may be associated with progression toward expanding saSDHs s, such as older age, bleeding tendency, and hematoma.561317) However, these results have been inconsistent. Additionally, different surgical methods for saSDH have been reported. The current study aimed to evaluate factors related to the development of expanding saSDHs and assess treatment methods used to relieve neurologic symptoms.
Go to : 
The medical records of 273 patients who underwent surgery between January 2006 and December 2012 due to a SDH were extracted from a trauma bank at our institution and reviewed. All patients with SDHs were diagnosed by a computed tomography (CT) scan at the emergency department and admitted to the hospital. Among the patients who were surgically treated, a saSDH was defined as follows: hematoma 4 to 21 days after head injury, clinical deterioration, and radiological expansion. Patients who underwent an operation for an acute SDH within 3 days of trauma and those with hematomas that evolved from acute SDHs more than 21 days later were excluded. Hematomas caused by a non-traumatic etiology were also excluded. Twenty-three patients fulfilled the aforementioned criteria for a saSDHs.
The following information was collected from medical records: demographic characteristics, co-morbid conditions, Glasgow Coma Scale (GCS) score at admission, usage of antiplatelet/anticoagulant agents at the time of admission, GCS at the time of surgery, surgical methods, and Glasgow Outcome Scale (GOS) on discharge. Radiologic data, such as hematoma density, midline shift, and thickness of the hematoma at the time of admission and just before the operation, were also analyzed. Hematoma density was classified into four types: hypodense, isodense, hyperdense, and mixed.
Go to : 
The mean GCS score of 23 patients on admission was 13±2, with good neurologic conditions; 14 were men and 9 were women. The mean age of the patients was 69 years (range, 23–79). Sixteen patients (69.6%) were older than 60 years, and 7 patients (30.4%) were younger than 60 (Table 1). The mechanisms of trauma were as follows: 15 involved slipping down, 3 involved falling from height, 4 had an unknown trauma history, and 1 involved assault. The frequently documented co-morbid conditions included hypertension (16 patients), diabetes mellitus (8 patients), liver cirrhosis (5 patients), coronary heart disease (3 patients), and previous history of a cerebrovascular accident (2 patients). Antiplatelet or anticoagulant therapy was provided to 12 patients (52.2%) (Table 1). One patient received both anticoagulant and antiplatelet agents. Elderly patients were more likely to receive antiplatelet therapy than younger patients.
The mean interval between trauma and surgery was 13.3 days (range, 4–20 days). At the time of surgery, all patients had experienced neurological deterioration. The mean GCS before surgery was 11. Regarding surgical methods, burr hole craniostomy with closed-system drainage was performed in 12 patients, twist-drill craniostomy with closedsystem drainage was performed in 10 patients, and craniotomy with hematoma removal was performed in 1 patient. In the craniotomy case, the patient preferred surgery under general anesthesia due to anxiety. The final GOS at discharge was 5, representing good recovery for all cases. No regression occurred after surgery.
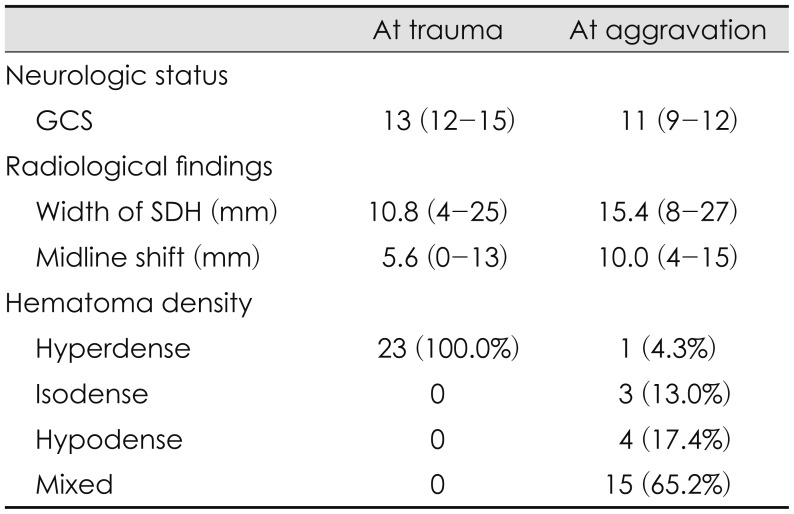
The saSDH occurred on the right side in 12 patients, the left side in 10 patients, and was bilateral in 1 patient. At the time of admission, the maximal thickness of the acute SDH and midline shift were 10.8 mm (range, 4–25) and 5.6 mm (range, 0–13), respectively. The hematoma density was hyperdense in all cases at admission (Table 2). At the time of the clinical deterioration, the maximal thickness of the hematoma increased to 15.4 mm (range, 8–27), and the midline shift was also aggravated to 10.0 mm (range, 4–15). The hematoma density was distributed more diversely: 15 in mixed form, 4 in hypodense form, 3 in isodense form, and 1 in hyperdense form (Table 2).
Go to : 
The natural course of initially non-operated acute SDHs is highly variable; it includes spontaneous resolution without any clinical symptoms, slow enlargement of the hematoma at the chronic stage, and rapid progression of the hematoma with clinical worsening in the subacute stage.517) There are clear differences between acute and chronic SDHs; however, the distinction between saSDHs and chronic SDHs is unclear. Therefore, saSDHs are sometimes reported as chronic SDHs because most patients in this category are treatable by burr hole surgery.5) The pathophysiology of a saSDH is not well-understood and may differ from that of a chronic SDH. Observation of saSDHs under craniotomy revealed a solid clot beneath the dura and showed that the liquid hematoma was closer to the brain and that the thin outer membrane histologically differed from that of chronic SDH.5) The clinical factors involved in the development of the saSDH were not the same as those involved in that of the chronic SDH.19) Therefore, we believe that saSDH is a distinct category of an expanding hematoma that evolves from an initially non-operative acute SDH.
Expanding saSDHs have been reported to occur in approximately 10% to 30% of acute SDHs treated conservatively.341719) In our study, the mean time of deterioration occurred around the 13th day after trauma, consistent with other studies.1219) Various surgical methods were proposed for the saSDH, including craniotomy, craniectomy, and burr hole procedures. The aim of surgery is to relieve the patients' symptoms. An expanding saSDH tends to occur more commonly in older patients with co-morbid medical conditions. Therefore, less invasive surgery is more suitable for a saSDH. In our study, all cases except one were treated with less invasive procedures, such as burr hole or twistdrill trephination with continuous catheter drainage. No revision surgery or recurrence was noted. Although catheter drainage would be preferred for a saSDH, craniotomy may be required for a hematoma that was inadequately drained.
Acute SDHs with a good neurological state and no mass effect can be safely managed without surgical intervention. A hematoma that exhibits neurologic deterioration and a mass effect during the closed observation leads to delayed surgical removal. It may be critical to identify the risk factors associated with a saSDH that expands from an initially non-operative acute SDH. However, the risk factors and pathogenesis have not been clearly defined. The initial volume of the hematoma and the degree of midline shift on the initial CT scan are believed to be associated with the delayed hematoma evacuation of an SDH.6) A larger hematoma can stretch out the bridging veins more severely and make them prone to tearing,10) resulting in hematoma progression. An alternative explanation is that persistent subdural fluid collection results in the development of a neomembrane, causing subsequent microbleeding. A larger initial hematoma may require more time for resolution and have a higher chance of developing a neomembrane.6) These theories suggest that the mechanism by which a hematoma expands and proceeds to the status of a saSDH (i.e., via the development of a neomembrane and microbleeding) is the same as that by which a chronic SDH does so.
However, the exact pathophysiologic mechanisms of an expanding saSDH may differ from those of a chronic SDH.121719) The time of surgery, which was usually around the 13th day after trauma in our data, may have been too early for the hematoma to resolve enough to be drained. The time required for hematoma expansion to cause neurologic deterioration was relatively short. We hypothesize that cerebrospinal fluid collection may play a role in expanding saSDHs. The operative results for seven cases of saSDHs ware reported by Morinaga et al.12) The operation found the outer membrane of the hematoma in only one of seven saSDHs, and the inner membrane was not identified in any case. The authors suggested that a lack of an outer membrane in cases with an saSDH prove this a different disease entity from a chronic SDH.12) Additionally, analysis of the hematoma contents showed low hemoglobin concentrations and a high level of methemoglobin. Thus, they believe that a saSDH is the result of subdural effusion in the subacute stage, and cerebrospinal fluid is considered responsible for the increase in mass volume.11217) Influx of cerebrospinal fluid into the subdural hematoma cavity from a torn arachnoid membrane and lysis of the subdural hematoma may be the principal mechanisms involved in rapidly expanding saSDHs. Impaired autoregulation in the hyperperfused state was proposed to explain the development of a saSDH.18) We did not experience any re-accumulation of the hematoma after surgery, which may explain the pathophysiologic differences between subacute and chronic SDHs. Magnetic resonance imaging plays an advanced role in differentiating subacute and chronic SDHs. In the subacute stage, diffusion-weighted imaging shows the two-layered mixture of high-signal intensity components, such as a solid clot, and low-signal intensity components, such as a liquefied hematoma.57)
Brain atrophy was proposed as a relevant factor in the expansion of SDHs from the acute to the chronic stage.41417) In our study, the mean age of patients with an expanding saSDH was 69 years, and this condition was most frequently observed in the elderly group. Given the continuing increase in the number of older individuals with non-operated acute SDHs, it is highly likely that clinicians will encounter saSDHs that require surgery. Acute SDHs with larger hematoma volumes that are not initially surgically treated should be carefully observed for progression into an expanding saSDH when they are seen in older patients.
Go to : 
An expanding saSDH, which exhibits neurologic deterioration and a mass effect, usually develops around the 13th day after head trauma. Burr hole or twist-drill trephination with closed-system drainage is enough to relieve the symptoms and mass effect. Older patients with large acute SDHs should be carefully monitored for development into an expanding SDH and undergo brain CT around the 13th day after head trauma.
Go to : 
References
1. Aoki N, Oikawa A, Sakai T. Symptomatic subacute subdural hematoma associated with cerebral hemispheric swelling and ischemia. Neurol Res. 1996; 18:145–149. PMID: 9162869.

2. Choi YH, Han SR, Lee CH, Choi CY, Sohn MJ, Lee CH. Delayed burr hole surgery in patients with acute subdural hematoma: Clinical analysis. J Korean Neurosurg Soc. 2017; 60:717–722. PMID: 29142632.
3. Godlewski B, Pawelczyk A, Pawelczyk T, Ceranowicz K, Wojdyn M, Radek M. Retrospective analysis of operative treatment of a series of 100 patients with subdural hematoma. Neurol Med Chir (Tokyo). 2013; 53:26–33. PMID: 23358166.

4. Izumihara A, Orita T, Tsurutani T, Kajiwara K. Natural course of non-operative cases of acute subdural hematoma: sequential computed tomographic study in the acute and subacute stages. No Shinkei Geka. 1997; 25:307–314. PMID: 9125713.
5. Izumihara A, Yamashita K, Murakami T. Acute subdural hematoma requiring surgery in the subacute or chronic stage. Neurol Med Chir (Tokyo). 2013; 53:323–328. PMID: 23708224.

6. Kim BJ, Park KJ, Park DH, Lim DJ, Kwon TH, Chung YG, et al. Risk factors of delayed surgical evacuation for initially nonoperative acute subdural hematomas following mild head injury. Acta Neurochir (Wien). 2014; 156:1605–1613. PMID: 24943910.

7. Kuwahara S, Fukuoka M, Koan Y, Miyake H, Ono Y, Moriki A, et al. Subdural hyperintense band on diffusion-weighted imaging of chronic subdural hematoma indicates bleeding from the outer membrane. Neurol Med Chir (Tokyo). 2005; 45:125–131. PMID: 15782003.

8. Kuwahara S, Fukuoka M, Koan Y, Miyake H, Ono Y, Moriki A, et al. Diffusion-weighted imaging of traumatic subdural hematoma in the subacute stage. Neurol Med Chir (Tokyo). 2005; 45:464–469. PMID: 16195646.
9. Lee KS. History of chronic subdural hematoma. Korean J Neurotrauma. 2015; 11:27–34. PMID: 27169062.

10. Lee KS, Shim JJ, Yoon SM, Doh JW, Yun IG, Bae HG. Acute-on-chronic subdural hematoma: Not uncommon events. J Korean Neurosurg Soc. 2011; 50:512–516. PMID: 22323938.

11. Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011; 114:72–76. PMID: 20868215.

12. Morinaga K, Matsumoto Y, Hayashi S, Omiya N, Mikami J, Sato H, et al. Subacute subdural hematoma: findings in CT, MRI and operations and review of onset mechanism. No Shinkei Geka. 1995; 23:213–216. PMID: 7700488.
13. Morinaga K, Matsumoto Y, Omiya N, Mikami J, Ueda M, Sato H, et al. Subacute subdural hematoma-report of 4 cases and a review of the literature. No To Shinkei. 1990; 42:131–136. PMID: 2192749.
14. Okumura Y, Shimomura T, Park YS. A study of acute subdural hematoma developing into hematoma with capsule formation. No Shinkei Geka. 1998; 26:691–698. PMID: 9743998.
15. Scotti G, Terbrugge K, Melançon D, Bélanger G. Evaluation of the age of subdural hematomas by computerized tomography. J Neurosurg. 1977; 47:311–315. PMID: 894336.

16. Singla A, Jacobsen WP, Yusupov IR, Carter DA. Subdural evacuating port system (SEPS)-minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg. 2013; 115:425–431. PMID: 22763191.

17. Son S, Yoo CJ, Lee SG, Kim EY, Park CW, Kim WK. Natural course of initially non-operated cases of acute subdural hematoma: the risk factors of hematoma progression. J Korean Neurosurg Soc. 2013; 54:211–219. PMID: 24278650.
18. Takeuchi S, Takasato Y, Otani N, Miyawaki H, Masaoka H, Hayakawa T, et al. Subacute subdural hematoma. Acta Neurochir Suppl. 2013; 118:143–146. PMID: 23564121.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download