Introduction
Burr hole craniostomy and closed-system drainage (BCD) have been widely used for the treatment of chronic subdural hematoma (SDH) or subdural hygroma. Despite a recurrence rate that cannot be overlooked, BCDs are widely used because they have are very simple procedures and can be performed even under local anesthesia with low complication rates.
561022) Nevertheless, as many neurosurgeons experience and has been reported in many case reports,
11,
14,
18,
19,
22) devastating complications such as acute intracranial hematoma or surgical error occur at a frequency that cannot be ignored.
Many studies on recurrence and inappropriate drainage associated with “failure to cure” have been carried out; however, the studies related to the complications of BCD have been very sporadic. Many neurosurgical centers appear to have unpublished experience of unexpected catastrophic postoperative complications.
21) The low complication rates due to publication bias make it difficult to provide appropriate information to patients and caregivers, subsequently creating possibilities for medico-legal problems. The objective of this study was to analyze all complications after BCDs in all consecutive patients in one institute since the opening of hospital.
Materials and Methods
A retrospective analysis was conducted for all patients who underwent BCD for presumed diagnosis of chronic SDH or subdural hygroma since the opening of one institution. This study was approved by the Institutional Review Board (KUH1070028). From August 2005 through September 2017, 452 BCDs were performed in 394 consecutive patients. The analysis of complications after BCD were limited to the first operation in 1 patient. Therefore, 51 BCDs for recurring lesions in 46 patients and repeat 6 BCDs for inappropriate drainage in 6 patients were excluded from the study. One patient underwent BCD for left chronic SDH in 2009 and BCD for right chronic SDH in 2014. We calculated this patient's data twice because we considered these two events to be independent. In total, 395 BCDs in 394 patients were included in this study.
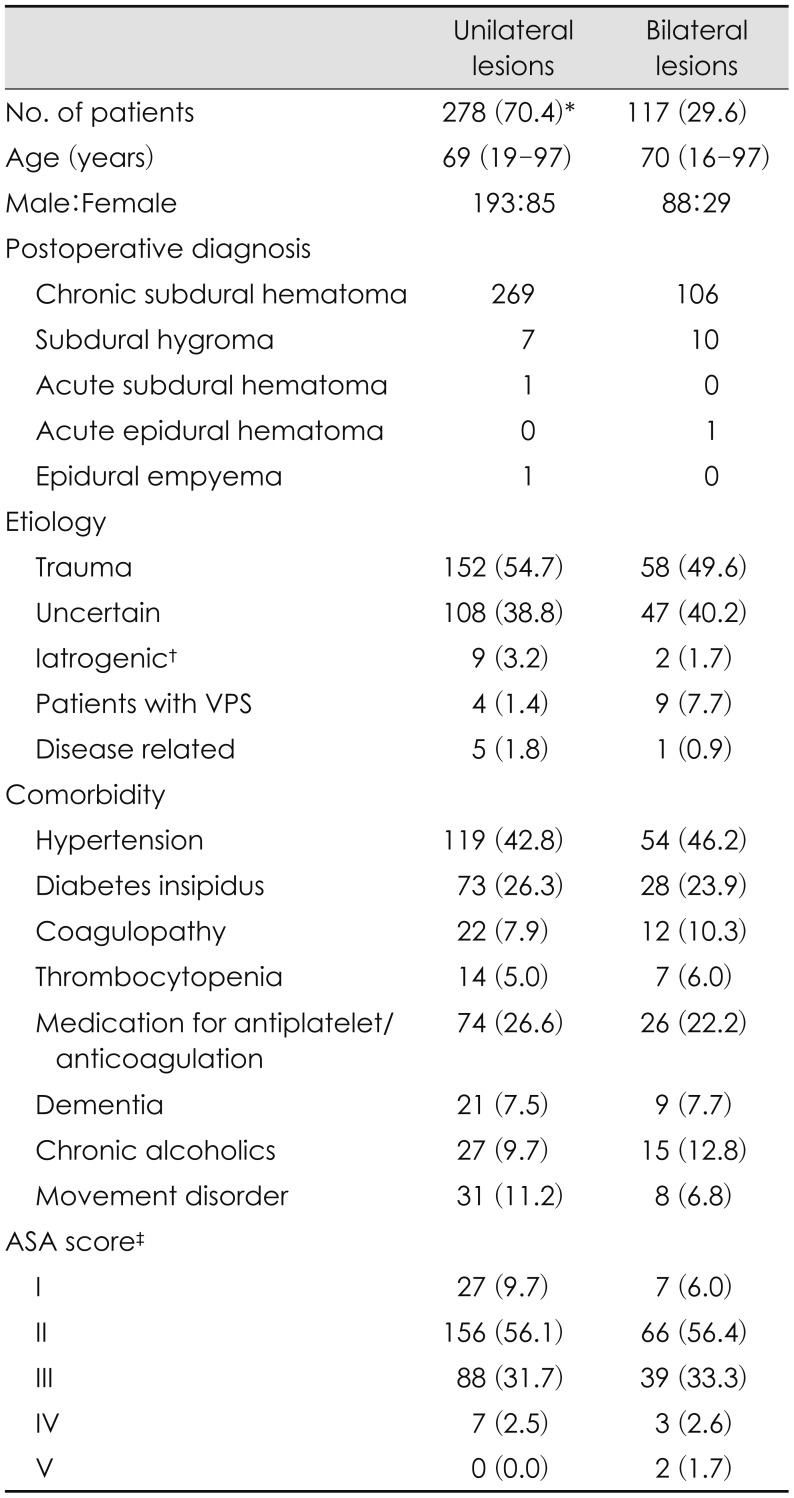
Table 1 shows the clinical characteristics of study population.
Operative complications were classified into surgical complications and nonsurgical complications. We classified operative complications into surgical complications and nonsurgical complications and did not analyze the recurrence of subdural lesions and reoperation after inappropriate drainage because these should be considered as a “failures to cure” rather than as operative complications.
3) Surgical complications were divided into acute intracranial hematoma, error in surgery or management, seizure and surgical site infection during the time interval between operation date and last follow-up date. Nonsurgical complications were defined as those requiring consultation or intervention by a specialist within 60 days of surgery.
The study group was composed of 281 men and 114 women, with a median age 69 years (range, 16–97). Etiologies were as follows: trauma was the most frequent with 210 patients (53.2%), followed by uncertain etiology in 155 patients (39.2%). Comorbidity status was assessed using the American Society of Anesthesiologists (ASA) physical status classification system. For patients who underwent general anesthesia we used the ASA score assessed by an anesthesiologist as described in the medical record. The ASA scores in patients who underwent local anesthesia were retrospectively evaluated using the medical records. ASA scores are summarized in
Table 1. ASA classes I and II were considered a low comorbidity status, and classes III, IV, and V were considered a high comorbidity status. Perioperative anti-epileptic drugs were used in 376 patients (95.2%). Monotherapy was used as the basis for the patients without seizure history. Valproic acid was used in 225 patients, levetiracetam was used in 122 patients, topiramate was used in 9 patients, and phenytoin was used in 7 patients. In 13 patients with seizure history, previously used antiepileptic drugs were applied during the perioperative period. The median duration of antiepileptic drug medication after surgery was 99 days, ranging from 0 days to 3,927 days. BCDs for unilateral chronic SDH and bilateral chronic SDH were performed in 269 patients and 106 patients, respectively. Seventeen patients underwent BCD for subdural hygroma. One patient underwent BCD for epidural hematoma, and another patient did so for acute SDH. One patient underwent BCD for presumed diagnosis of chronic SDH that was diagnosed as epidural empyema postoperatively. In cases of unilateral lesions, unilateral one burr hole craniostomies were performed in 206 patients (74.1%), and two burr hole craniostomies were performed in 72 patients (25.9%). Intraoperative saline irrigation was performed in 141 patients (35.7%). The method of anesthesia was made according to surgeon's preference, patient's general condition and compliance. General anesthesia was performed in 268 patients (67.8%), and local anesthesia was performed in 107 patients (27.1%).
The number of surgical complications was insufficient to use statistical methods; therefore, statistical analyses were only performed for nonsurgical complications. Statistical analyses were performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The relationship between categorical variables and presence or absence of nonsurgical complications was assessed by the χ2 test. Finally, variables with p<0.2 were analyzed using a binary logistic regression model. Nonsurgical complications were analyzed using the following variables: age (≥70 years old vs. <70 years old), anesthesia method (general anesthesia vs. local anesthesia), ASA class (I, II vs. III, IV, V), and hospital stays before surgery (≤7 days vs. >7 days).
Results
Intracranial hemorrhagic complications
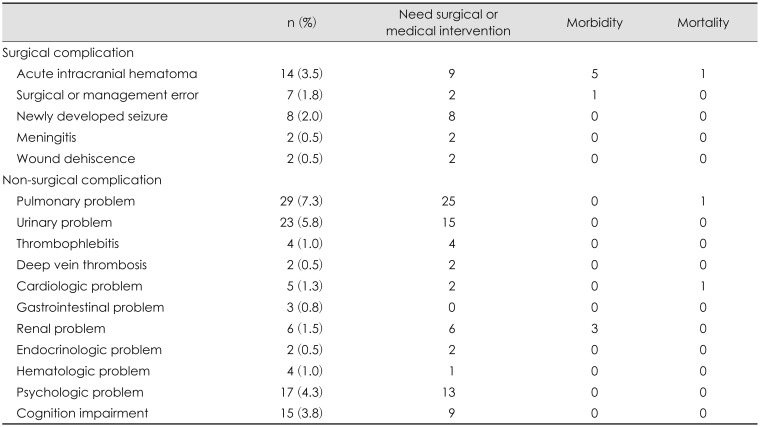
All surgical and nonsurgical complications reported in our study were reviewed and shown in
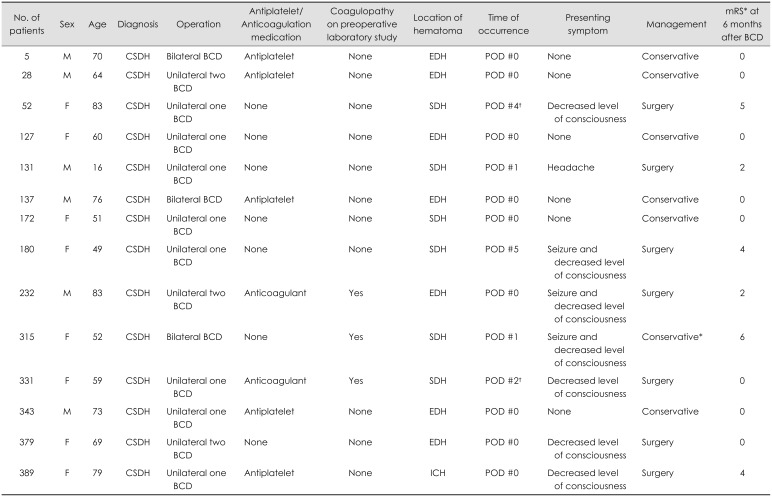
Table 2. In 14 of the 395 (3.5%) patients, acute intracranial hematomas were newly developed after BCDS.
Table 3 shows the clinic-radiologic profiles and clinical outcomes of patients with intracranial hemorrhagic complications. The location of intracranial hemorrhage was “epidural hematoma” in 7 patients, “SDH” in 6 patients, and “intracerebral hematoma” in 1 patient. All acute epidural hematomas occurred adjacent to the burr hole site and were confirmed on immediate postoperative computed tomography (CT) scan. Two of these patients showed decreased levels of consciousness and underwent emergent craniotomy and hematoma evacuation; they recovered without significant neurological deficits. By contrast, the time of onset of acute SDHs varied. Only 1 patient was identified in an immediate postoperative CT scan, and he was managed conservatively; he recovered without neurological deficits. Three patients with drainage catheters in situ developed acute SDH, 2 patients on day 1 and 1 patient on day 5 after BCD. In the other 2 patients, acute SDH developed immediately after the removal of drainage catheter. Four patients underwent craniectomy and SDH evacuation; however, 3 patients had moderate-to-severe disability. For the other patient with acute SDH, the family refused surgery and patient died because of brain herniation. In 1 patient, small intracerebral hematoma was occurred near the burr hole site. This patient underwent emergent craniotomy at 2 days after BCD due to hematoma expansion and development of neurological symptoms.
Surgery or management errors
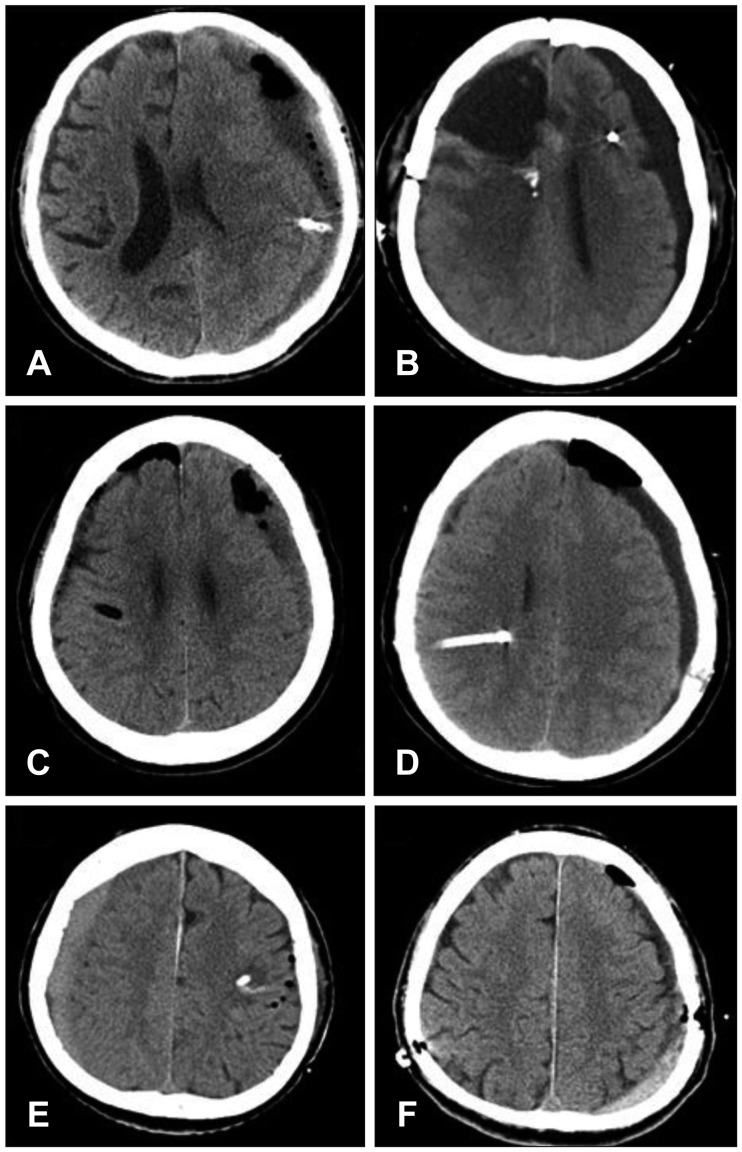
Drainage catheter malposition in the brain parenchyma occurred in 4 cases. In all 4 cases, burr hole craniostomy was performed in the parietal bone: the drainage catheter was inserted upwardly in 3 cases (
Figure 1A, C, and D) and downwardly in 1 case (
Figure 1B). Reoperation was performed in only 1 patient (
Figure 1A), and there were no additional neurological deficits associated with catheter malposition.
Opposite-side surgery occurred in two cases. In one case (
Figure 1F), the surgeon was aware of the opposite side during surgery. However, in another case (
Figure 1E), the surgical procedures proceeded on the opposite side, and the drainage catheter was placed in the brain parenchyma. Postoperatively, the patient developed right-sided facial palsy.
In one case, accidental catheter withdrawal occurred during the transfer from the operating room to the ward. Reoperation was performed in this patient.
Seizure
Of the 386 patients without a history of seizure before BCD, 8 patients had seizures after BCD. All the patients were on prophylactic anti-epileptic drug medication, and 7 of the patients were on medication at the time of seizure. Of the 8 patients with seizures after BCD, 3 patients had seizure due to acute intracranial hematoma after BCD; 3 patients had seizure without neuroimaging changes, 1 patient at 3 days, one at 13 days, and one at 15 days after BCD; 1 developed seizures three months after BCD due to recurrence of chronic SDH; and 1 had a seizure 2 years after BCD due to dural arteriovenous fistula.
In 8 patients with a history of seizure related to subdural lesions, 5 were seizure-free after BCDs and 2 had seizures during the postoperative hospital stay; however, since then, there has been no seizure, and discontinuation of medication was achieved at 110 and 274 days, respectively; 1 patient who had seizure during the postoperative hospital stay expired due to cardiac arrest at 38 days after BCD.
Four patients who had previously seizures due to other diseases had no changes in seizure frequency and have continued on antiepileptic drug medication.
Nonsurgical complications
Eighty-eight patients (22.3%) suffered from nonsurgical complications after BCD.
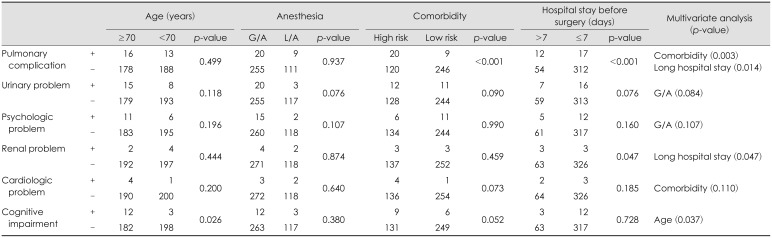
Table 2 shows the incidence of nonsurgical complications. Pulmonary problems (7.3%) were the most common nonsurgical complications followed by urinary problems (5.8%), psychologic problems (4.3%) and cognitive impairments (3.8%). Two deaths occurred associated with nonsurgical complications during the hospital stays. The causes of death in 2 patients were pneumonia and sudden cardiac arrest. The risk factors for nonsurgical complications were statistically analyzed (
Table 4). High comorbidity was associated with pulmonary complications (
p=0.003). Long hospital stay before surgery was associated with pulmonary complications (
p=0.014) and renal complications (
p=0.047). Old age was a risk factor for cognitive impairment after BCD (
p=0.037). The method of anesthesia was not associated with any nonsurgical complications.
Discussion
Chronic SDH is one of the most commonly treated lesions by neurosurgeons.
2491516) BCD is known to be relatively fast and safe and is most commonly used for its treatment.
14) There have been many studies on treatment failures of BCD that have been referred to as recurrences. Nevertheless, studies focusing on the complications after BCD are very rare, and most studies only reported the complications briefly. In addition, article types reporting catastrophic complications such as acute intracranial hematoma and surgical error are usually case reports, considered to have a low level of evidence; therefore, it is almost impossible to estimate the prevalence of these complications.
We found only one article
14) focusing on complications after BCD in a literature search of PubMed. Rohde et al.
14) performed a retrospective study of complications after BCD in 376 patients with chronic SDH. According to this report, intracranial hemorrhage occurred in 13 patients (3.4%), of which 8 cases (2.1%) were intracerebral hemorrhage and 5 (1.3%) were epidural hematoma. In our study, 1 patient (0.2%) suffered from intracerebral hematoma, and epidural hematoma occurred in 7 patients (1.7%). Epidural hematoma may be caused by tearing of the middle meningeal artery or venous injury of the dural sinuses or diploic veins.
12) These are the only possible complications related to stress of local forces. According to reported cases, epidural hematoma after BCD in patients with chronic SDH are highly correlated with shear forces in surgical procedure or detachment between dura and the cranium.
712172021) In accordance with previous reports, all epidural hematomas in our series were revealed on immediate postoperative CT scans and were located at near the burr hole site.
Acute SDH occurred in 6 patients (1.6%) in our series; however, this type of hematoma did not occur in any patient according to the previously mentioned study.
14) Unlike epidural hematoma, the onset of acute SDH varied. Only one case of acute SDH was detected on immediate postoperative CT scans, and others developed during hematoma drainage or immediately after the removal of drainage catheter. Unpredictability in the development of acute SDH creates the need to for patients and caregivers to be informed of the risks, and great caution and close observation should be exercised in postoperative management, especially after the removal of drainage catheters.
There has been only one case report of procedure-related complications; Pavlov et al.
13) reported parenchymal malposition of a drainage catheter. Our study showed that similar complications occurred in 4 patients (
Figure 1A–D). In all cases, burr hole craniostomy was performed in the parietal bone, and in 3 of these, the catheter was advanced in the upward direction. We speculate that acute angle formation between the parenchymal surface and the drainage catheter that is made by parietal burr hole craniostomy, supine position and advancement to upward direction, appears to contribute to parenchymal malposition of the drainage catheter.
Human errors such as opposite-side surgery should never occur; unfortunately, these errors have always existed because they are an inherent part of human behavior.
1) Systems to maximize patient safety are fundamental to reduce human errors.
1) In our institute, opposite-side surgery has not occurred in the neurosurgical practice since August 2015 when a triple-checking system was introduced: a patient's surgical information is verified by a nurse, an anesthesiologist and the surgeons.
In the present study, newly developed seizures after BCD occurred in 8 patients (2.0%). These results are relatively lower than those of previous reports that showed seizure incidences between 2.2% and 13.6%.
1114181922) We presumed that the use of prophylactic antiepileptic drugs in a high proportion of patients contributed the relatively low rate of seizure after BCD. Five patients (62.5%) among those with newly developed seizures after BCD had provoked seizures due to new intracranial lesions. Therefore, a prompt radiological evaluation should be performed in patients with newly developed seizures after BCD.
Patients with chronic SDH are often elderly and have medical comorbidities that are often associated with multiple trauma. Therefore, it is not surprising that many nonsurgical complications occurred after BCD. The most common nonsurgical complications were pulmonary problems (n=29, 7.3%), followed by urinary problems (n=23, 5.8%) and psychologic problems (n=17, 4.3%); 2 patients died due to nonsurgical complications (0.5%). According to multivariate analysis of risk factors, long hospital stays significantly correlated with pulmonary complications (
p=0.014). Bedridden patients who were more likely to stay in the hospital longer than ambulatory patients were more vulnerable to pulmonary complications such as pneumonia. Thorough preoperative risk evaluations and meticulous postoperative care such as early ambulation are mandatory to reduce nonsurgical complications as much as possible. Many researchers asserts that BCDs should be performed under local anesthesia to reduce nonsurgical complications.
814) However, our results revealed that the method of anesthesia was not associated with any nonsurgical complications, though there was a tendency for a slight increase in urinary and psychologic problems after general anesthesia. The possibility of anesthesia-related complications appears to be further reduced in the future due to the advancement of anesthesia techniques and drugs. Further studies will be required to ascertain the proper method of anesthesia for BCD, considering not only the anesthesia-related complications but also the psychological factors.
Our study has some limitations stemming from retrospective design. First, there was no definite agreement as to what operative complications should be counted. There is controversy regarding whether such complications as acute bleeding and seizures should be regarded as complications of chronic SDH itself rather than complications of BCD. We analyzed most of the nonsurgical complications depending on consultation. Therefore, it was difficult to analyze complications by severity grading; minor complications such as atelectasis may have been overlooked, and the overall incidence of complications may have been underestimated. Second, BCD was performed by 13 neurosurgeons, and there were some differences in surgical strategy, including the number of burr holes or saline irrigations among individuals. Although there are some heterogeneities such as surgical skills and patient characteristics, we believe that inclusion of all consecutive patients without exclusion criteria minimized bias and made it possible to evaluate the complications after BCD in the most objective and frank manner.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download