This article has been
cited by other articles in ScienceCentral.
Abstract
The rapid spontaneous resolution of an acute epidural hematoma (EDH) has rarely been reported. A possible mechanism of spontaneous resolution is egress of the hematoma into the subgaleal space through a skull fracture. We report a case of rapid redistribution of an acute EDH in a 37-year-old man who had a malignant peripheral nerve sheath tumor of the skull and who slipped and fell when going to the bathroom. A huge EDH without a skull fracture developed in the left parieto-occipital area. The acute EDH was completely alleviated and a newly developed intracerebral hematoma was found on a brain computed tomography scan that was acquired the day after the trauma. Given these findings, a fractured skull and increased pressure in the intradural area may have been the mechanisms underlying the redistribution of the hematoma.
Go to :

Keywords: Epidural hematoma, Malignant nerve sheath tumor, Neurofibromatosis, Resolution, Skull fracture
Introduction
An acute epidural hematoma (EDH) is a frequently encountered traumatic lesion in patients with closed head injuries. It is usually accompanied by a skull fracture, and the prognosis is better than other traumatic intracranial hematomas.
236) A symptomatic acute EDH is typically treated with emergent surgical decompression to relieve intracranial pressure. An asymptomatic acute EDH frequently enlarges, and thus repeat computed tomography (CT) imaging is recommended.
10)
Rapid spontaneous resolution of an acute EDH is a rare phenomenon.
39) It probably occurs as the hematoma egresses out of the subgaleal space through an overlying fracture.
36) We report a case of a huge traumatic acute EDH without a skull fracture in a patient with a malignant peripheral nerve sheath tumor (MPNST), which completely disappeared. The possible mechanism of this unexpected phenomenon is discussed.
Go to :

Case Report
A 37-year-old man had a scalp mass on the occipital area surgically excised at an outside clinic 10 years ago. In November 2013, he came to the neurosurgery clinic of our hospital for recurrence of the occipital mass (
Figure 1A). He had numerous café-au-lait spots on the chest and abdomen and axillary freckles. Neurofibromatosis type 1 was confirmed with skin manifestations. The occipital mass was totally excised and revealed to be a benign neurofibroma. A new mass was found on the left occipito-temporal area in March 2015 (
Figure 1B). Pathology revealed an MPNST. He underwent conformal radiotherapy on the bony lesions (54 cGy/27 fx). Local recurrence in the occipital area developed in November 2016, and the recurrent lesion was resected and confirmed to be an MPNST. A right pleural mass developed in January 2017 (
Figure 1C). Palliative chemotherapy (ifosfamide 2,500 mg/m
2 on days 1–3 plus doxorubicin 60 mg/m
2 on day 1 in 4 week intervals) was administered, and eight chemotherapy cycles were completed.
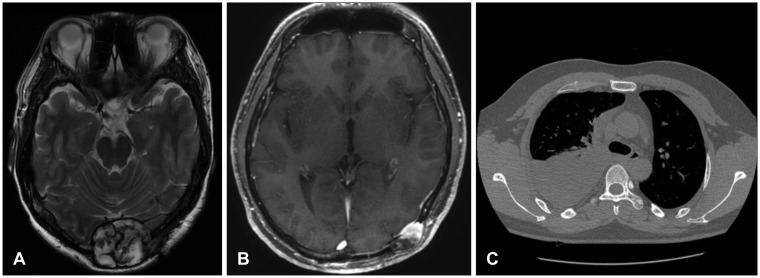 | FIGURE 1Radiological images related to the tumor. (A) A round heterogeneous mass on the midline of the occipital skull was detected in a T2-weighted axial image. It was a benign neurofibroma. (B) A new mass developed in the left occipito-temporal scalp at 16 months after the midline tumor was excised. This was confirmed to be a malignant peripheral nerve sheath tumor. The skull and scalp lesions were irradiated. (C) A pleural mass was found in the right chest at 3 years after the scalp malignancy was diagnosed. Palliative chemotherapy was administered.
|
At the last chemotherapy session, he complained of headache, neck pain, and shoulder pain and began to take opioid analgesics. He was admitted to our hospital and underwent tomotherapy for cervical spine cancer. He had no definite motor weakness or limitation in the activities of daily living. He then fell when getting up to go to the bathroom in the early morning, hitting the occipital part of his head and shoulder. He showed stuporous consciousness and a Glasgow Coma Scale score of 7. His right pupil was blown to 8 mm without a light reflex, and the left arm was paralytic. A large lentiform epidural mass and a newly developed brain shift to the left was evident on a brain CT scan (
Figure 2A and B). A lytic skull lesion and scalp tumor were noted in the right parietal area, which were previously known pathological conditions. No skull fracture was found (
Figure 2C and D). Levels of hemoglobin and platelets were 9.0 g/dL and 192,000/µL, respectively. The coagulation profile was normal with a 1.18 international normalized ratio coagulation index prothrombin time and a 25 seconds activated partial thromboplastin time. The CT findings were compatible with a huge acute EDH on the right parietal side and the effect of the mass, but epidural invasion of the malignant tumor was not absolutely ruled out at that time. His family did not want surgical decompression because of the incurable state of his malignancy. He was transferred to the intensive care unit, where fluid therapy including intravenous mannitol was administered. His consciousness improved with spontaneous eye-opening and he was able to follow motor commands in the afternoon. However, the right pupil remained unreactive to light. Neurological deterioration followed, and the next morning he was semicomatose. The huge epidural mass had completely disappeared and a new large intracerebral hematoma on the putamen and an intraventricular hemorrhage were found on a brain CT scan (
Figure 2E). A brain shift to the left was still evident, and the cortical sulci were more effaced. The scalp had expanded slightly compared to the previous CT scan (
Figure 2F). He died of the head trauma on day 7.
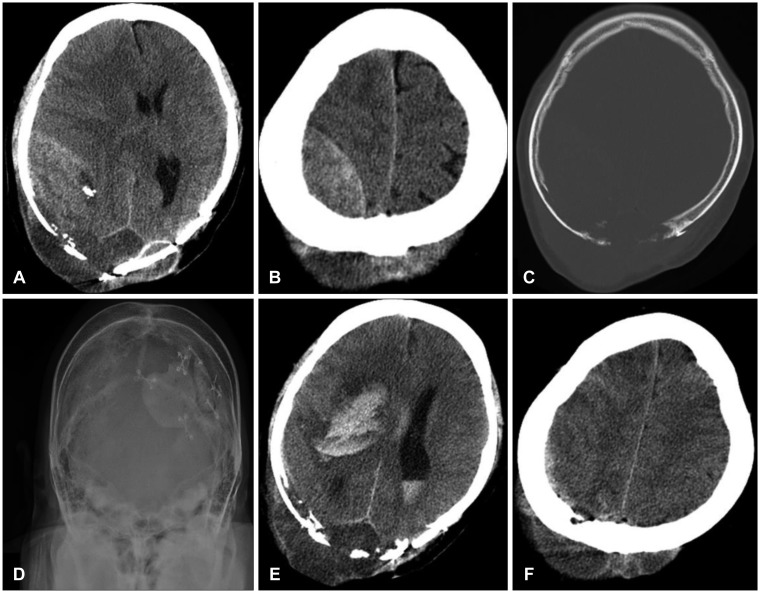 | FIGURE 2Radiological images of the epidural hematoma. (A, B) A huge epidural hematoma was found in the right temporo-parietal area on a brain computed tomography (CT) scan taken immediately after the trauma. (C) Small bone chips were evident in the hematoma. Osteolysis of the inner skull and a bony defect on the occipital skull were detected. (D) No skull fractures were observed. (E) A brain CT scan the day after the trauma revealed that the hematoma had disappeared and an intracerebral hematoma had newly developed on the putamen. (F) Subgaleal swelling, as revealed by a follow-up CT scan, was minimal.
|
Go to :

Discussion
Spontaneous resolution of an acute EDH rarely occurs during the early period of head trauma. This phenomenon usually occurs in childhood.
469) Rapid resolution occurs when the EDH is redistributed to the subgaleal space. Pulsatility of the intracranial contents provides the necessary force to drive blood through the fracture, even if intracranial pressure is not elevated.
19) The loose adhesion of the galea to the skull in childhood may be one of the factors in this rare situation. It tends to resolve with a relatively small parietal hematoma.
36)
An acute EDH develops after a direct impact to the head and is usually accompanied by an overlying skull fracture. Bleeding originates from the meningeal artery and/or the diploic space around the fracture line. Although most acute EDHs are symptomatic and require immediate surgical evacuation, a small acute EDH with no or few neurological symptoms is usually treated conservatively.
57) Asymptomatic acute EDHs tend to grow during the early period after a trauma.
810) Thus, repeated CT scans should be conducted during the early stages after trauma. The possibility of an acute EDH occurring should be considered.
Fractures play critical roles in redistributing the EDH into the extracranial space.
369) The roles of intracranial pressure and the pressure gradient are unclear. Distinctive findings of previous reported cases included fractures and relatively small hematomas that were large enough to be evacuated, whereas no skull fracture was found in our case. Our patient had an osteolytic skull bone and a hole on the midline close to the superior sagittal sinus instead of a skull fracture. The EDH probably came from the diploic vein in the area of osteolysis. The intracerebral hematoma elevated intracranial pressure sufficiently to push out the EDH. The EDH may then have been driven into the subgaleal space through the hole in the skull in the osteolytic area. We do not know exactly why the intracerebral hematoma occurred in our case.
However, redistribution of the hematoma into the subgaleal space does not explain the disappearance of the huge EDH. The patient had long-term scalp cancer and underwent radiotherapy to the skull and scalp. Thus, we did not expect a space in the subgaleal area. A follow-up CT scan showed little swelling of the scalp. We speculate that the diploic vein, which was exposed to the epidural space due to destruction of the inner table of the skull, and the superior sagittal sinus, which may have been eroded by the cancer, may have been involved in the spontaneous resolution of the EDH. The increased intradural pressure may have pushed the hematoma into the venous circulation through a probable venous opening in the diploic vein or the sagittal sinus.
Go to :

Conclusion
Rapid spontaneous resolution of an acute EDH without a skull fracture can occur in a patient with a skull malignancy. Increased intradural pressure may cause the EDH to disappear via the venous system.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download