Abstract
We report the case of a patient with organized chronic subdural hematoma (OCSH) that was treated with craniotomy. A 72-year-old man was admitted with a complaint of a drowsy mental status after a generalized tonic-clonic seizure. A brain computed tomography scan acquired at a local hospital revealed a large chronic subdural hematoma (CSDH) in the left frontoparietal lobe. The patient had not experienced head trauma and had been taking clopidogrel due to angina. A neurosurgeon at the local hospital performed single burr hole trephination in the left frontal bone and drained some of the hematoma. Brain magnetic resonance imaging performed upon transfer to our hospital showed a large OCSH with a midline shift to the right side, revealing a low, heterogeneous signal on T2-weighted images (WI) and an isodense signal on T1-WI. We performed craniotomy and membranectomy to achieve adequate decompression and expansion of the brain. Following this, the patient recovered completely. Our findings support that neurosurgeons should consider the possibility of organization of a CSDH when selecting a diagnosis and treatment plan.
Among chronic subdural hematomas (CSDHs), organized CSDH (OCSH) is rare.14910) A common CSDH can be treated by burr hole trephination and drainage of the hematoma; however, OCSH is not usually treated by burr hole drainage and it is treated by craniotomy and membranectomy. Physicians should carefully suspect OCSH when CSDH is diagnosed because usual burr hole drainage cannot effectively remove the organized hematoma and the thick membrane due to solidity. Here, we report a case of OCSH treated with craniotomy and membranectomy and discuss the clinical course.
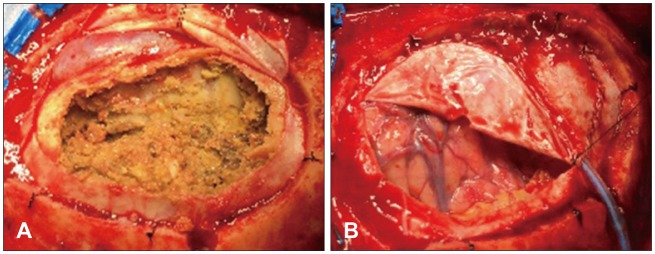
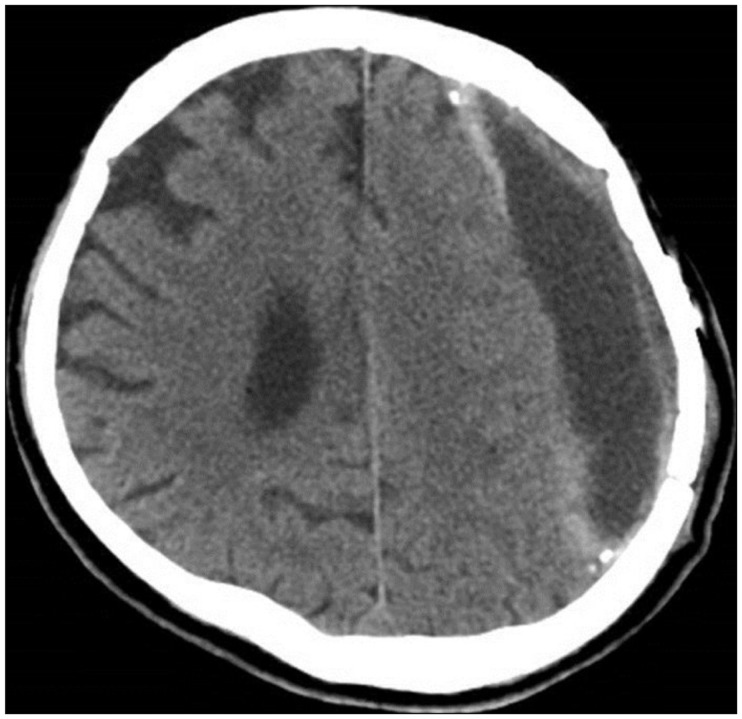
A 72-year-old man was referred from a local hospital due to impaired consciousness after a generalized tonic-clonic seizure. He had been diagnosed with a large amount of CSDH in the left frontoparietal lobe and a midline shift to the right hemisphere (Figure 1A). A neurosurgeon at the local hospital had performed burr hole trephination in left frontal bone and he had failed to drain the hematoma sufficiently (Figure 1B and C). The hematoma was made up of yellow, mud-like material, and it had been misdiagnosed as subdural empyema in the operative room. The doctor immediately transferred the patient to our hospital in order to treat subdural empyema. A neurological examination at admission revealed drowsy mentality with a Glasgow Coma Scale score of 13. Hemiparesis of the right arm and leg was not detected. He could not recall any incident of head trauma. He had hypertension, diabetes, angina, and he had been taking clopidogrel for treatment of angina. A brain computed tomography (CT) scan showed many high density areas in the hematoma and the membrane, compared to usual CSDH. Focal calcifications were found in the membrane. Brain magnetic resonance imaging (MRI) showed a large amount of CSDH with a low, heterogeneous signal on T2-weighted images (WIs), an isodense signal on T1-WIs, and a low, homogeneous signal on diffusion-WIs (Figure 2). There was no dense enhancement as with a subdural empyema. The membrane surrounding the hematoma was enhanced in a line. He was diagnosed with OCSH based on brain images and previous operative findings. We decided to treat by performing craniotomy, followed by removal of the organized hematoma and the thick membrane. We performed a frontoparietal craniotomy and incised the dura mater. After dural incision, we removed a thick, calcified outer membrane. Under the outer membrane, we found a large amount of yellow, sticky, mud-like organized hematoma with a thick inner membrane (Figure 3A), and no hemorrhagic fluid. The thick, hard, and calcified inner membrane covered the brain cortex. The inner membrane blocked the expansion even after adequate removal of OCSH. We gently performed inner membranectomy and confirmed pulsation in the normal brain cortex (Figure 3B). Pathologic examination revealed an old hematoma with focal calcification and chronic inflammation. Immediate postoperative brain CT showed adequate removal of the OCSH and the inner membrane. Clopidogrel was started as an antiplatelet therapy 1 week after the craniotomy after confirming the absence of acute hemorrhage. He was discharged from the hospital on clopidogrel, and he was scheduled to receive a neurological follow-up. A brain CT scan at discharge showed subdural fluid collection in the subdural space. One month after surgery, brain CT showed no recurrence of CSDH with subdural fluid collection (Figure 4). He was in an alert state on follow-up examination, and he recovered completely.
Typical CSDH is liquid hematoma. In some cases, the ininternal structure of the hematoma can be multiseptated or multilayered. OCSH is defined as CSDH, has a thick membrane with multiseptations, and forms an encapsulated area with a solid consistency.1) The incidence of OCSH is low, and its pathogenesis is unclear. This form of CSDH is different from typical CSDH which consists of a fibrous capsule made up of inner and outer membranes filled with bloody fluid.16) It is not known why some CSDHs become organized against hyperfibrinolytic activity known to be the pathogenesis of typical CSDH. The neomembrane in OCSH is similar to granulation tissue.5) Fibrous material slowly increase in volume, forming a solid hematoma in which the inner and outer membranes tend to fuse completely.2) Callovini et al.1) reported that a prevalent minor head injury was present in 60% of OCSH patients, while in the remaining patients, history of a traumatic event was unclear. No underlying risk factors were found in 35% of the patients. Clinical symptoms of OCSH are variable and they include headache, alteration of consciousness, motor weakness, aphasia, and seizure. Mostly, there are only minor symptoms and there may be no neurologic deficits. Overlooking minor symptoms of CSDH leads to a long standing hematoma.
OCSH is diagnosed by enhanced MRI or CT. Hematomas have a multiseptated or multistaged complex structures with prevalent solid components. Compared to a typical CSDH with multiple layers, OCSH has more excessive formation of solid membranes and non-liquefied hematomas with different hemorrhagic lesions.1)
Treatment of OCSH is removal of both the organized hematoma and the membrane. However, craniotomy and membranectomy for OCSH are rare. Rocchi et al.8) experienced 14 patients with OCSH, representing 5.8% of the 243 patients treated for CSDHs. Nine patients underwent craniotomy and membranectomy as the second line of management and five patients underwent initial craniotomy and membranectomy. They suggest that MRI detection of thick and extensive membranes or a solid clot with mass effect makes it necessary to perform an immediate craniotomy to remove OCSH. Isobe et al.4) reported about the primary operation of CSDH in 535 patients. Of these, 6 patients were diagnosed with OCSH and they underwent craniotomy. Shrestha et al.9) performed craniotomy with radical membranectomy in 3 of the 100 patients with CSDH. Craniotomy with membranectomy was performed in one case as second surgery after single burr hole drainage because of recurrence of CSDH. One patient developed a seizure after craniotomy. Craniotomy and removal of the membranes still carry high rates of mortality and morbidity.3) Aggressive membranectomy may induce a postoperative seizure, brain contusion, and hemorrhage. These complications result from traction injury during the attempt to remove the membranes adhering tightly to the cortex. Oda et al.7) suggested that the inner membrane should be preserved and left intact on the brain surface after removal of only the calcified layer. We recommend that craniotomy and membranectomy for OCSH be considered after failed burr hole drainage to facilitate brain re-expansion and prevent recurrence.
The prognosis for OCSH is highly variable, but it often remains poor, depending on the extent of the damage, the timing of surgery, and the presence of comorbidity. OCSH often result in a critical manifestation, including seizure and motor weakness. Prompt removal of a large amount of hematoma is essential to relieve the critical state condition and to improve the prognosis. A failed burr hole surgery can delay proper craniotomy and worsen the prognosis. The rate of OCSH in patients with CSDH is low because CSDH is detected in the early stages before deterioration of neurologic symptoms. However, if detection of CSDH is delayed, CSDH may develop into OCSH, requiring an enlarged craniotomy. In addition, an organized hematoma can compress the brain parenchyma for a long time and cause convulsions, as observed in the present case. Enlarged craniotomy may be critical in elderly patients because the blood oozing from the margin of the incised membrane can cause an acute subdural hematoma or recurrence of CSDH. Callovini et al.1) reported that in one case (3%), intraventricular and subarachnoid hemorrhage occurred which led to death. Two cases (6%) presented with a hemorrhagic stroke ipsilateral to the OCSH. Also, many elderly patients take medications like antiplatelet and anticoagulation agents for cerebrovascular and cardiac diseases and they have a coagulopathy in the liver and renal disease. Additional surgery is often required after the primary operation and it leads to a poor prognosis.
References
1. Callovini GM, Bolognini A, Callovini G, Gammone V. Primary enlarged craniotomy in organized chronic subdural hematomas. Neurol Med Chir (Tokyo). 2014; 54:349–356. PMID: 24305027.

2. Fujioka M, Okuchi K, Miyamoto S, Sakaki T, Tsunoda S, Iwasaki S. Bilateral organized chronic subdural haematomas: high field magnetic resonance images and histological considerations. Acta Neurochir (Wien). 1994; 131:265–269. PMID: 7754833.

3. Hellwig D, Heinze S, Riegel T, Benes L. Neuroendoscopic treatment of loculated chronic subdural hematoma. Neurosurg Clin N Am. 2000; 11:525–534. PMID: 10918025.

4. Isobe N, Sato H, Murakami T, Kurokawa Y, Seyama G, Oki S. Six cases of organized chronic subdural hematoma. No Shinkei Geka. 2008; 36:1115–1120. PMID: 19086442.
5. Kawano N, Endo M, Saito M, Nakayama K, Beppu T. Origin and pathological significance of smooth muscle cells and myofibroblasts in the subdural neomembrane. Neurol Med Chir (Tokyo). 1986; 26:361–368. PMID: 2429215.

6. Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004; 61:523–527. discussion 527-528. PMID: 15165784.

7. Oda S, Shimoda M, Hoshikawa K, Shiramizu H, Matsumae M. Organized chronic subdural haematoma with a thick calcified inner membrane successfully treated by surgery: a case report. Tokai J Exp Clin Med. 2010; 35:85–88. PMID: 21319032.
8. Rocchi G, Caroli E, Salvati M, Delfini R. Membranectomy in organized chronic subdural hematomas: indications and technical notes. Surg Neurol. 2007; 67:374–380. PMID: 17350406.

9. Shrestha P, Pant B, Shrestha P, Rajbhandari P. Organized subdural hematoma with thick membrane in chronic subdural hematoma. JNMA J Nepal Med Assoc. 2012; 52:1–5. PMID: 23279765.

10. Suyama T, Ubagai R, Inui T, Tominaga S. Chronic subdural hematoma organized over a short period of time with spontaneous intracranial hypotension: a case report. Brain Nerve. 2012; 64:855–860. PMID: 22764357.
FIGURE 1
Brain computed tomography (CT) scan (A) showed chronic subdural hematoma containing many high density areas in the hematoma and the membrane in the left frontoparietal lobe with a midline shift. After burr hole surgery, brain CT axial (B) and coronal (C) scans demonstrated insufficient drainage of the hematoma through a single burr hole.
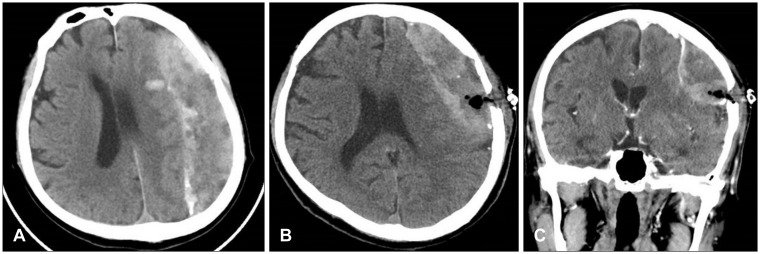
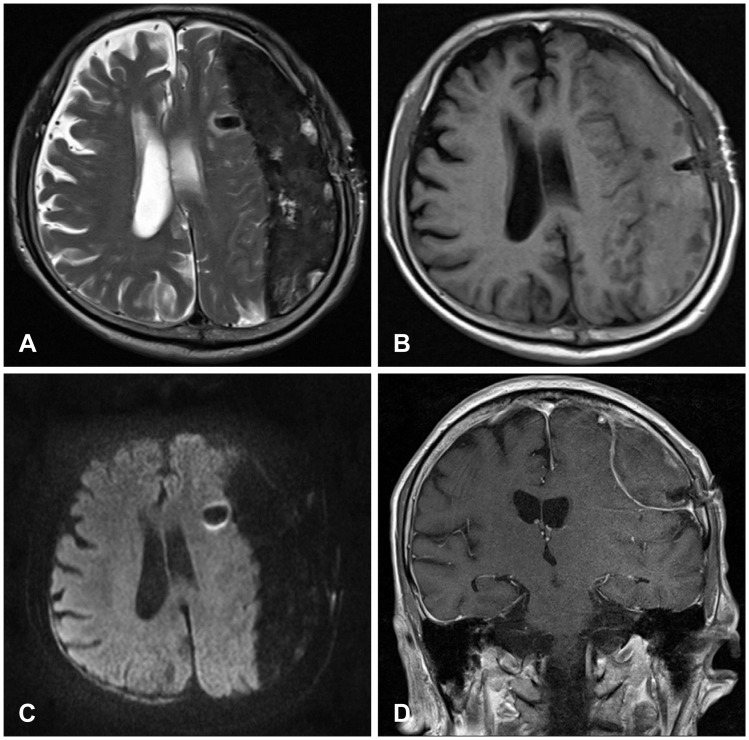
FIGURE 2
Brain magnetic resonance imaging showed a large amount of chronic subdural hematoma with a low, heterogeneous signal on T2-weighted images (WI) (A), an isodense signal on T1-WI (B), and a low, homogeneous signal on diffusion-WI (C). There was no strong enhancement and the membrane surrounding the hematoma was enhanced in a line (D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download