Abstract
We reconstructed an extensive vulvar defect with the internal pudendal artery and the anterior obturator artery perforator flap and compared the two types of flaps. A 57-year-old female patient had a 15×13 cm2 defect on both sides around the vagina after vulvectomy. Due to the injury in the internal pudendal artery, we used the internal pudendal artery perforator on the right side, and the anterior obturator artery perforator flap on the left side. The internal pudendal artery perforator flap could be rotated easily 120 degrees toward the vulvar defect. However, as the anterior obturator artery perforator flap was difficult to rotate, reconstruction was performed by rotation and advancement. The anterior obturator artery perforator flap is a viable alternative method that can be used when it is difficult to use the internal pudendal artery perforator flap during vulvar reconstruction.
Vulvar cancer is rare and usually requires tumor ablation surgery, which results in a need for functional and aesthetic reconstruction12. When the defect is small, reconstruction can be done with primary closure, local flap, and skin graft. When the defect is large, reconstruction can be done using the pedicled deep inferior epigastric artery perforator (DIEP) flap, the gracillis musculocutaneous flap, and free flaps1. As the lotus pedal flap and the perforator flap are increasingly recognized, excellent functional and aesthetic outcomes are reported by performing reconstruction with a thin perforator flap around the vulva3.
The pedal flap based on the internal pudendal artery perforator, various types of internal pudendal artery perforator flaps, or other options can be used2. The internal pudendal artery perforator flap is designed based on the vessel running along the vulva margin, which is safe and reliable, but if the tumor is large and extensive excision needs to be done, another surgical method must be selected because of artery injury2. In this case, it was difficult to use the internal pudendal artery perforator, and reconstruction was carried out using the anterior obturator artery perforator at the more distal site of the medial thigh. Therefore, the authors compare the two different types of flaps with our experience.
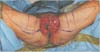
A 57-year-old female patient had a 0.8×0.5 cm2 mass at the lower 1/3 of vulva labia majora. In the biopsy and pelvis computed tomography, she was diagnosed with stage IIIB squamous cell carcinoma of the vulva. In the Department of Gynecology at this hospital, the tumor was diagnosed as malignant on the frozen biopsy performed during operation. While vulvectomy, bilateral inguino-femoral lymph node dissection, pelvic and para-aortic lymph node dissection were underway, the excision range of the vulva became larger, and primary closure or local flap coverage became difficult. Thus, an emergency operation was performed for reconstruction. After wide vulvectomy, a 15×13 cm2 defect was observed on both sides around the vagina and urethra opening, while the anus was intact (Fig. 1).
Because of the emergency operation, we could not evaluate the vascular condition around the vulva. For reconstruction of the vulva defect, a fasciocutaneous flap based on the internal pudendal artery perforator was designed, and if the use of perforator flap proved difficult, the obturator artery based gracillis musculocutaneous V-Y advancement flap was prepared (Fig. 1). The internal pudendal arteries on both sides were checked using a hand-held doppler, but due to the vessel injury caused by the wide tumor excision, the internal pudendal artery was not found on the left side. Reconstruction was planned using the internal pudendal artery perforator for the right side and the anterior obturator artery perforator for the left side.
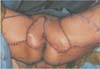
We made the skin incision around the right posterior medial thigh, and dissected the skin flap via supra-fascial plane while we tried to find the perforator from the proximal side to the distal side of the medial thigh. The skin perforator was present in the inside of the lower margin of the vascular triangle from the ischial tuberosity to apex of the coccyx and came from the internal pudendal artery. We elevated the 15×7 cm2 sized perforator flap for the defect of the right side vulva. The flap thickness around the internal pudendal artery perforator was 1.9 cm, the elevated flap was rotated clockwise by 120 degrees, and tension-free closure was conducted for the vulvar defect (Fig. 2A). The donor site could be closed primarily.
For the left vulva defect, suprafascial dissection was done from the proximal thigh to the distal over the gracillis muscle, and a proper size of perforator was found, specifically the anterior obturator artery perforator. The subcutaneous fat tissue thickness over the gracillis muscle was 3.5 cm. We elevated the 18×8 cm2 sized perforator flap for the left vulva defect. The position of the perforator was very deep and more distal than the position of the internal pudendal artery perforator (Fig. 2B). The elevated flap was advanced and rotated counterclockwise by 90 degrees. Tension-free primary closure could be done for the donor site (Fig. 3).
Two weeks later, there was partial necrosis on the right side flap, but it healed spontaneously. There were no other complications. Five months later, volume reduction of the both flaps multiple Z-plasty were performed due to flap bulkiness and scar contracture (Fig. 4).
The vulvar region is an organ that represents female identity, and when reconstructing the defect occurring after wide surgical resection of vulva cancer, the functional and aesthetic aspects of the vagina and urethra, as well as the coverage of the soft tissue defect, must be considered. The wide range of reconstructive options for vulvar reconstruction includes skin grafts, skin flaps, fasciocutaneous flaps and myocutaneous flaps124.
In reconstruction for extensive vulvar defects, medial thigh V-Y advancement flap, the gracillis musculocutaneous flap, the pedicled DIEP flap, and free flap are often selected, but these methods pose the disadvantage of low aesthetic and functional satisfaction due to thick flap thickness after reconstruction3. The lotus pedal flap and the internal pudendal artery perforator flap centered on the gluteal fold are excellent alternatives in the aesthetic and functional aspects, because thin covering is possible, allow a better anatomical reconstruction, respect inguinal–crural crease and leave minimal scars in folds56. Hashimoto et al.2 reported that these flaps are quick, safe, sensitive and more aesthetically effective. As an alternative flap, O'Dey et al.7 presented the anterior obturator artery perforator flap. They said that the anterior obturator artery perforator pierces the gracilis muscle proximally on the level of its thin aponeurosis as an indirect or musculocutaneous perforator near to the inferior pubic ramus. They suggest that the anterior obturator artery perforator flap could be thinner because both the septocutaneous and musculocutaneous anterior obturator perforators extend nearly perpendicularly to the subdermal plexus. In our case, because of the injury on the internal pudendal artery, it was difficult to use the internal pudendal artery perforator flap. Based on the assessment that a reliable flap had to be selected, the obturator artery perforator flap was used first, having the medial thigh V-Y advancement flap, the anterior gracillis musculocutaneous flap and free flaps as the second choice8910.
Since two different types of perforator flaps were performed on either side, advantages and disadvantages could be compared (Table 1). The position of the anterior obturator artery perforator was more distal than that of the internal pudendal artery perforator, and the skin thickness over the gracillis was thicker than that of the internal pudendal artery perforator. In addition, due to the thickness, dissection time of the anterior obturator artery perforator was longer without initial flap thinning procedure on both sides. The position of the perforator was very deep and more distal than the position of the internal pudendal artery perforator. The arc of rotation of the flap was limited due to the thick flap, deep placed perforator, insecurity of the vascular pedicle torsion or kinking8. Because propeller pattern rotation is difficult, advancement and rotation is considered a safer method rather than rotation alone.
The internal pudendal artery perforator flap was thinner and rotation was easier than the anterior obturator artery perforator flap. However, the reconstruction method with the anterior obturator perforator flap was safe, and can be a good alternative choice for vulvar reconstruction when it is difficult to use the internal pudendal artery perforator flap.
Figures and Tables
References
1. Höckel M, Dornhöfer N. Vulvovaginal reconstruction for neoplastic disease. Lancet Oncol. 2008; 9:559–568.

2. Hashimoto I, Abe Y, Nakanishi H. The internal pudendal artery perforator flap: free-style pedicle perforator flaps for vulva, vagina, and buttock reconstruction. Plast Reconstr Surg. 2014; 133:924–933.
3. Bodin F, Weitbruch D, Seigle-Murandi F, Volkmar P, Bruant-Rodier C, Rodier JF. Vulvar reconstruction by a “supra-fascial” lotus petal flap after surgery for malignancies. Gynecol Oncol. 2012; 125:610–613.

4. Al-Benna S, Tzakas E. Postablative reconstruction of vulvar defects with local fasciocutaneous flaps and superficial fascial system repair. Arch Gynecol Obstet. 2012; 286:443–448.

5. Sawada M, Kimata Y, Kasamatsu T, et al. Versatile lotus petal flap for vulvoperineal reconstruction after gynecological ablative surgery. Gynecol Oncol. 2004; 95:330–335.

6. Tan BK, Kang GC, Tay EH, Por YC. Subunit principle of vulvar reconstruction: algorithm and outcomes. Arch Plast Surg. 2014; 41:379–386.

7. O'Dey DM, Bozkurt A, Pallua N. The anterior Obturator Artery Perforator (aOAP) flap: surgical anatomy and application of a method for vulvar reconstruction. Gynecol Oncol. 2010; 119:526–530.
8. Lazzaro L, Guarneri GF, Rampino , et al. Vulvar reconstruction using a “V-Y” fascio-cutaneous gluteal flap: a valid reconstructive alternative in post-oncological loss of substance. Arch Gynecol Obstet. 2010; 282:521–527.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download