Abstract
The posterior interosseous artery (PIA) flap is one of the options for hand and upper extremity reconstruction. It does not sacrifice the main arteries of the hand, the radial and ulnar arteries and could be used even when either artery was damaged. The PIA is a branch of the common interosseous artery, which is about 1 cm in distance from the ulnar artery, and runs down longitudinally in the intermuscular septum between the extensor carpi ulnaris and extensor digiti minimi. PIA appears to be relatively constant in position, and pro a reliable blood supply in the posterior aspect of the forearm. The PIA flap is reliable in its designs, even to making it possible to close the donor site primarily. It provides not only a thin, pliable coverage of the hand and upper extremity, but also a neurosensory flap. Technically, the dissection of the PIA pedicle along its course needs a high learning curve, because it might present the risk of venous congestion, ischemic flap necrosis, and injury to the PIN. Although the flap dissection seems to be difficult, it still offers increased versatility in reconstructions of the hand, foot, and upper extremity.
Since its first description in 1988 by Zancolli and Angrigiani1, the posterior interosseous artery (PIA) flap is one of the options for hand and upper extremity reconstruction. As a type B fasciocutaneous flap according to the Lamberty and Cormack classification2, it is also called the dorsal or posterior forearm flap34. It could be used as proximally-based for elbow coverage, and distally-based island flap for hand and wrist reconstruction, in addition to the free flap. It does not sacrifice the main arteries of the hand, the radial and ulnar arteries; and this must be its biggest advantage. It could also be used even when either artery was damaged, or palmar arches were absent.
The PIA flap provides an appropriate thickness and good skin texture for the hand and elbow reconstruction. Although anatomic variations of PIA are very common, it has been constantly located in the intermuscular septum between the extensor digiti minimi (EDM) and extensor carpi ulnaris (ECU) muscle (Fig. 1), and is rich in cutaneous branches. The retrograde PIA (rPIA) flap has been originally reported for adduction contracture of the thumb and extensive defects of the hand dorsum1. Besides the first web space, the reach of the rPIA flap could enable reconstruction of the dorsal and palmar aspects of the hand, including the metacarpophalangeal joints and the dorsum of the thumb. The anastomosis between the anterior interosseous artery (AIA) and the PIA at the proximal part of the wrist joint allows the rPIA flap to be available, even in the presence of extensive vascular damage of the hand5.
The versatility and reliability of PIA flap has been proved by many surgeons136789, and it has gained widespread acceptance and popularity. The PIA flap could also be performed as a free flap with higher success rate3 when the pedicled flap is problematic, due to the absence of connection with the AIA, or injury of the distal forearm10. Otherwise, the AIA perforator flap could also be a backup procedure for the pedicled PIA flap511.
In this paper, we thoroughly review the PIA flap papers through Pubmed search, and consider the reliability of the PIA flap in reconstruction of the composite tissue defects in the hand and upper extremity.
From March 2003 to April 2009, a retrospective review was performed on the patients who underwent rPIA flap at MS Jaegeon Hospital. For each patients, the data including sex, age, etiology, the size and region of the defect, flap size, operation results, and complication were collected.
The forearm skin is supplied by perforating subcutaneous and musculocutaneous arteries that originate from the radial, ulnar, anterior, and posterior interosseous arteries2. The PIA is a branch of the common interosseous artery, which is about 1 cm in distance from the ulnar artery, and runs down longitudinally in the intermuscular septum between the ECU and EDM. This vascular arrangement forms the basis for an island fasciocutaneous flap that can be based either proximally or distally.
Although variations and absence of the PIA commonly occur in the mid forearm61213, the PIA appears to be relatively constant in position, and provides a reliable blood supply in the posterior aspect of the forearm. While ending on the dorsal aspect of the carpus, it participates in the dorsal carpal arch. Generally, there are two dominant connections between the AIA and PIA; one proximal (distal border of the supinator muscle), and the other distal (distal part of the interosseous space at the wrist level)1. The latter anastomosis with dorsal branch of the AIA is usually found 2 cm above the distal radioulnar joint (DRUJ). Many anatomical studies101415 have also noted the PIA anastomoses with the perforating branch of the AIA, the dorsal carpal arch, and the vascular plexus surrounding the ulnar head at the level of the wrist. Those are believed to be critical for the successful rPIA flap4. Zaidenberg et al.16 used a dorsal intercarpal arch through the 5th extensor compartment artery as a pivot point of the PIA flap, and extended the distal reach of the PIA flap to even the fingertips. Pahl and Schmidt14 noted an anastomosis between the PIA and a perforating branch of the AIA in the middle third of the forearm in 20%. Ringformations of the palmar and the dorsal branch of the AIA occurred in 5%.
Absence of anastomosis between the PIA and the recurrent dorsal branch of the AIA was reported with an incidence of 1% to 3%8. Additionally, the angiosome of the middle third of the PIA seems to be the weakest part of the blood supply, due to hypoplasia or aplasia1018. Narrowing and termination of the PIA in the middle third of forearm has also been reported11217.
The PIA provides 5 to 13 septocutaneous perforators supplying the dorsum of the forearm, which rise vertically, and reach the overlying skin.6 Lu et al.18 found that the PIA gives off 3 to 9 septocutaneous perforators at the middle third and 2 to 5 at the distal third of the forearm. Therefore, the middle third of the forearm was called the “zone of security” of harvesting the skin paddle. Sun et al.19 investigated the cutaneous perforators of PIA using microscopy techniques, and noted that the PIA has two main clusters of perforators in the middle and distal fifths of the forearm. Through this anatomical basis, they simplified the dissection by the PIA pedicle cutaneous branch-chain perforator flap. Costa and Soutar20 divided the PIA perforators into 3 distinct patterns, and noted that the most common type was the multiple small branches arising at 1 to 2 cm intervals along the total length of the PIA.
The PIA is closely related to the last motor branches of the posterior interosseous nerve (PIN), which gives a sensory branch to the carpus and the motor branches for the deep layer muscles on the dorsal forearm. The distal branches of PIN to the extensor pollicis longus and extensor indicis proprius muscle are known to be most vulnerable during the dissection of the PIA pedicle distally4.
The distally-based PIA flap has been widely reported for coverage on a defect on the fingers (Fig. 2), dorsum of the hand, and the first interdigital web (Fig. 3, 4). However, its anatomical variations of the PIA make it difficult to dissect the flap. Technically, the dissection of the PIA pedicle along its course needs a high learning curve, because it might present the risk of venous congestion, ischemic flap necrosis, and injury to the PIN.
The flap axis is drawn from the lateral epicondyle of the humerus to the DRUJ in full pronation. The donor zone lies in the middle of the axis, due to the presence of the dominant skin perforators in that area. Using the Doppler, the main perforators of PIA can be located 2 cm distal to the midpoint of the axis, or 9 cm distal to the lateral epicondyle of the humerus. Due to the wide variation of the PIA, preoperative Doppler chasing for the PIA is mandatory.
The direction of the dissection is dependent on the surgeon'xs choice. Many surgeons suggested the proximodistal dissection of the flap, because the absence of anastomotic arc with AIA is rare122122. The approach from the distal to the proximal makes it easier to identify the distal anastomosis at first. The PIA lies relatively superficial in the distal half, and lies deep in the proximal half, which makes the distal-to-proximal dissection feasible. However, dissection of the PIA in the distal third of the forearm is tedious and difficult. If the proper anastomotic arc is not revealed, the surgery could be switched to the other options, such as a free flap, or the AIA perforator flap3.
Originally Angrigiani et al.12 raised the flap in the suprafascial plane from its radial margin. However, we recommend the starting of the dissection from the ulnar border, because of its simplicity in identifying the ECU muscle belly. After coming across the main perforators, we switched the dissection for the radial side in the ulnar direction towards the intermuscular septum between the ECU and EDM. To improve the venous drainage, a large amount of fascia might be included throughout the length of the pedicle. The dissection should be continued, until the fascial plexus and the branches to the distal end of the ulna are met. The difficulty in dissection results from its anatomical variations, and unreliability in case of traumatic history at the distal forearm.
Ascertaining the presence of distal PIA communication with the AIA is the first step in flap elevation. However, we do not recommend the isolation of this anastomotic arc. The pedicle should be kept as broad as possible, to prevent venous congestion. If this anastomosis is not present, this procedure should be switched immediately to the other options, such as the PIA free flap. Otherwise, the patient has only the linear incision scars on the forearm. In addition to the dorsal recurrent branch of the AIA, which is also called the perforating branch of the AIA, the other possible connections with the dorsal carpal arch should be kept in mind during the dissection.
The pivot point is marked between 2.5 cm proximal to the radial edge of the ulnar head. The PIA could be dissected and ligated just proximal to the main perforators. Temporary clamping of the proximal end of the PIA is helpful, to confirm the safe reverse flow from the AIA. The average length of the pedicle in the PIA flap was reported to be 7.1 cm4. The pedicle should include 2 cm of fascia and the intermuscular septum between the ECU and EDM. Its venae comitantes run along the intermuscular septum. The flap is inset by tunneling or splitting the skin bridge between the pivot point and the defect for the transfer of the pedicle. The subcutaneous tunnel should be wide enough to avoid the venous congestion due to the compression of the pedicle. After elevating the rPIA flap, the proximal skin paddle is rotated 180 degrees as a perforator-based propeller flap. When the flap is raised with 3 to 4 cm width, the donor site can be closed primarily.
Care must be taken to preserve the deep branches of the radial nerve and its branches. The PIN is dissected off the PIA proximally. PIA perforators have always been found just distal to this motor branch, so that a retrograde flap can safely be raised with preservation of this nerve branch. If a neurosensory flap is required, the posterior antebrachial cutaneous nerve might be included with the PIA flap10.
A total of 21 cases in 20 patients were included. The mean age of the patients was 38 years (range, 10-65 years); 18 male patients and 2 female patients. All the cases were caused by trauma and the defects ranged in size from 16 to 90 cm2. The defect areas were 1st web space in 4 cases, amputated thumb in 6 cases, hand dorsum in 7 cases, palm in 1 case, and wrist in 3 cases (Fig. 2, 3, 4). In 15 cases, either the radial or ulnar artery was not traced preoperatively. All the flaps were survived completely except 2 cases of complications; 1 in hematoma and 1 in infection.
With various forearm flaps based on the radial/ulnar artery, the PIA/AIA could be used for the reconstruction of the hand and upper extremity4. The flaps using the former two arteries are criticized for breaking the main circulation to the hand, which results in cold intolerance, hand stiffness, and varying degree of sensory loss23. To that point, PIA/AIA flaps are theoretically much more attractive, with lower donor site morbidity, and higher patient acceptance2425.
As the PIA flap was initially introduced in a distally based retrograde pattern1, it has been suitable for the repair of various soft tissue defects in the fingers (Fig. 2), wrist, palm, hand dorsum, and first web space (Fig. 3, 4)117262728. The proximally-based PIA adipofascial flap was also used to prevent the recurrence of synostosis of the elbow joint and forearm29. They have many advantages of thin, soft, and pliable skin with good color- and texturematch with the hand. The rPIA flap depends on retrograde flow from a complex vascular arcade at the wrist level, and its frequent anatomical variations and technically demanding procedure still make hand surgeons hesitate to choose it as a first line in hand reconstruction. Therefore, wide knowledge of the anatomical variations, meticulous surgical technique for flap dissection, and a proper learning curve are the key points for the successful flap elevation and survival. Interestingly, according to Vögelin et al.30, there was no statistical correlation between anatomical variations and complications or PIA flap loss. Generally, the rPIA flap is avoided in patients with deep scar tissue, such as electrical burns. However, Inoue and Taylor31 reported the successful rPIA flap using previously burned skin.
Regarding the mechanism of venous drainage of the distally-based, retrograde, reverse flap, Lin et al.32 explained the “crossover” and “bypass” effects between the two venae comitantes as deep venous system. The “bypass pattern” of the collateral branches of each vein enables the reverse drainage of venous blood, even though the valves of the vein are intact.
Venous congestion is the main concern of the rPIA flap, which has been reported to range 3% to 37%131333. The proximal axis of rotation of the rPIA flap limits the distal reach to the MCP joint level, which imposes a venous congestion and ischemic necrosis of the flap. It seems to be related to the width of the pedicle, and the narrowness of the subcutaneous tunnel. To avoid subcutaneous tunnel compression, Zaidenberg et al.16 recommended detaching the pedicle with the septum included, and splitting the skin bridge between the rotation point and the recipient. Puri et al.33 also took a wide strip of fascia and subcutaneous tissue in the rPIA flap. We usually recommend leaving at least 0.5 cm of fibro-fatty sleeve on the pedicle. Özalp et al.25 included the subcutaneous vein as a salvage pathway, instead of including a fascial strip. Reyad et al.34 decreased the inflow in the flap by decreasing the number of included perforators, and included only 1 perforator in the rPIA flap less than 40 cm2. In addition, the distal reach of the rPIA flap has also been improved by more distal dissection along the transverse anastomotic branch17. Bowstringing it across the wrist keeping it in extension33, splitting of the skin bridge between the pivot point and the defect35, using an exteriorized pedicle36, and the extended skin flap937, have all been evolved to make the rPIA flap more reliable.
To increase the rPIA flap survival, Acharya et al.35 avoided the dissection of the anastomotic arc between the AIA and PIA, while Chen et al.38 suggested the additional venous anastomosis and change into a free flap. Reyad et al.34 designed the racquet-shaped flap to avoid tunneling, and to add more superficial veins. Fong and Chew39 suggest the incorporation of as many perforators to the flap as possible, and fascia extension to allow greater reach of the flap, even to the distal interphalangeal (DIP) joint. Cavadas9 extended the pedicle length of the anterograde PIA flap, by designing the skin flap more distally at the dorsal wrist crease level. Tiengo et al.40 elongated the rPIA pedicle of 24% using the ligation of the AIA 5 mm before the emergence of the ramus communicans.
The PIA flap can be performed as an adipofascial33, osteofascicutaneous41, or a vascularized tendon or bone graft33. Suzuki et al.42 applied the adipofascial turnover perforator flap based on the PIA. Multi-paddled PIA flap has been reported to reconstruct the multiple different hand subunits843. Li et al.43 used the multilobed flap method for multi-finger soft tissue defect reconstruction, which decreases the risk of synanastomosis. Sungpet and Patradul44 introduced a new flap based on the longitudinal fascial branches of the PIA, which could reduce the risk of surgical failure due to irregular perforator distributions. They reserved subcutaneous veins that entered into each paddle in the case of venous congestion.
Inoue and Taylor31 found that the forearm was divided into three angiosomal units or cutaneous vascular territories. The middle third of the dorsum was coincident with that of Zancolli and Angrigiani1's report. Prasad and Balakrishnan45 emphasized the role of the interosseous recurrent artery perforator in the PIA flap, which arises from the proximal quarter of the dorsal forearm. This could extend the range of PIA flap coverage over the proximal third of the forearm. They applied the reverse-flow YV pedicle extension to raise a flap on a branch of a Y-like vascular bifurcation46. Pedicled perforator flaps on the posterolateral mid-forearm have been investigated with the advantage of no need of microsurgical expertise, one surgical field, shorter operation time, and good matching in skin color and texture4748.
To increase the versatility of the rPIA flap, Park et al.10 refined its use as a direct-flow free flap. They applied it as a fasciocutaneous, fasciocutaneous-facia, and a fasciaonly free flap in the hand and foot reconstructions. According to Costa and Soutar20 and Costa et al.41, the diameter of the PIA is 1.5 mm (range, 1.2 to 2.1 mm), which enables adequate successful microvascular anastomosis. The PIA perforator free flap was also used in intermediate-sized defects of the hand and foot that are too large to be covered by a local flap, or too small for a first-line perforator flap4950. In spite of the technical demand, it provides a minimally invasive procedure for donor and recipient sites. The drawback of the short pedicle could be overcome by perforator-to-perforator supermicrosurgery.
In addition to the aforementioned various situations, the rPIA flap has been also reported in revision surgery after carpal tunnel release to wrap around the neurolyzed median nerve3051. Shibata et al.3 combined the reversed pedicled PIA flap with the lateral arm flap to cover a large defect. They anastomosed the posterior radial collateral artery to an artery in the recipient site to enhance blood circulation. Costa et al.41 and Martin et al.46 elevated the vascularized PIA pedicled ulnar segment for the thumb reconstruction and infected forearm nonunion separately. Pagnotta et al.52 also treated ulnar nonunion with the vascularized distal radius graft based on the PIA.
Cavadas53 and Liu et al.54 include the posterior cutaneous nerve of the forearm to the PIA flap, called a neurocutaneous flap, and this perineural arterial network might have contributed to its viability in the absence of the distal artery. Park et al.10 suggested that the terminal part of the PIN could be used as a vascularized nerve graft, when harvested with the accompanying PIA.
The great merit of the PIA flap is that it does not sacrifice the main arteries to the hand. It is reliable in its designs, even to making it possible to close the donor site primarily. The PIA flap not only provides a thin, pliable coverage of the hand and upper extremity, but also a neurosensory flap with the posterior antebrachial cutaneous nerve. Although the flap dissection seems to be tedious and difficult due to its frequent anatomical variations, it still offers increased versatility in reconstructions of the hand, foot, and upper extremity.
Figures and Tables
Fig. 1
The schema of reverse PIA flap. PIA: posterior interosseous artery, EDM: extensor digit minimi, ECU: extensor carpi ulnaris, AIA: anterior interosseous artery, IRA: interosseous recurrent artery.
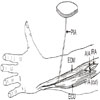
Fig. 2
A 49-year-old male patient had an amputation at the mid-shaft level of the 1st metacarpal bone. Four months later, the rPIA flap provided an additional base for a toe-to- thumb transfer. (A) Preoperative view. (B) Flap elevation. (C) Coverage of the remnant thumb with rPIA flap. (D) Postoperative view. rPIA: retrograde posterior interosseous artery.

References
1. Zancolli EA, Angrigiani C. Posterior interosseous island forearm flap. J Hand Surg Br. 1988; 13:130–135.

2. Gavaskar AS. Posterior interosseous artery flap for resurfacing posttraumatic soft tissue defects of the hand. Hand (N Y). 2010; 5:397–402.

3. Shibata M, Iwabuchi Y, Kubota S, Matsuzaki H. Comparison of free and reversed pedicled posterior interosseous cutaneous flaps. Plast Reconstr Surg. 1997; 99:791–802.

4. Bayon P, Pho RW. Anatomical basis of dorsal forearm flap. Based on posterior interosseous vessels. J Hand Surg Br. 1988; 13:435–439.

5. Chen HC, Tang YB, Chuang D, Wei FC, Noordhoff MS. Microvascular free posterior interosseous flap and a comparison with the pedicled posterior interosseous flap. Ann Plast Surg. 1996; 36:542–550.

6. Costa H, Pinto A, Zenha H. The posterior interosseous flap - a prime technique in hand reconstruction. The experience of 100 anatomical dissections and 102 clinical cases. J Plast Reconstr Aesthet Surg. 2007; 60:740–747.

7. Fujiwara M, Kawakatsu M, Yoshida Y, Sumiya A. Modified posterior interosseous flap in hand reconstruction. Tech Hand Up Extrem Surg. 2003; 7:102–109.

8. Zhang YX, Qian Y, Pu Z, et al. Reverse bipaddle posterior interosseous artery perforator flap. Plast Reconstr Surg. 2013; 131:552e–562e.

9. Cavadas PC. Posterior interosseous free flap with extended pedicle for hand reconstruction. Plast Reconstr Surg. 2001; 108:897–901.

10. Park JJ, Kim JS, Chung JI. Posterior interosseous free flap: various types. Plast Reconstr Surg. 1997; 100:1186–1197. discussion 1198-9.

11. Giunta R, Lukas B. Impossible harvest of the posterior interosseous artery flap: a report of an individualised salvage procedure. Br J Plast Surg. 1998; 51:642–645.

12. Angrigiani C, Grilli D, Dominikow D, Zancolli EA. Posterior interosseous reverse forearm flap: experience with 80 consecutive cases. Plast Reconstr Surg. 1993; 92:285–293.
13. Penteado CV, Masquelet AC, Chevrel JP. The anatomic basis of the fascio-cutaneous flap of the posterior interosseous artery. Surg Radiol Anat. 1986; 8:209–215.

14. Pahl S, Schmidt HM. [Clinical anatomy of the interosseous arteries of the forearm]. Handchir Mikrochir Plast Chir. 1994; 26:246–250. German.
15. Sheetz KK, Bishop AT, Berger RA. The arterial blood supply of the distal radius and ulna and its potential use in vascularized pedicled bone grafts. J Hand Surg Am. 1995; 20:902–914.

16. Zaidenberg EE, Farias-Cisneros E, Pastrana MJ, Zaidenberg CR. Extended posterior interosseous artery flap: anatomical and clinical study. J Hand Surg Am. 2017; 42:182–189.

18. Lu LJ, Gong X, Liu ZG, Zhang ZX. Antebrachial reverse island flap with pedicle of posterior interosseous artery: a report of 90 cases. Br J Plast Surg. 2004; 57:645–652.

19. Sun C, Wang YL, Ding ZH, et al. Anatomical basis of a proximal fasciocutaneous extension of the distal-based posterior interosseous flap that allows exclusion of the proximal posterior interosseous artery. J Plast Reconstr Aesthet Surg. 2015; 68:17–25.

20. Costa H, Soutar DS. The distally based island posterior interosseous flap. Br J Plast Surg. 1988; 41:221–227.

21. Balakrishnan G, Kumar BS, Hussain SA. Reverse-flow posterior interosseous artery flap revisited. Plast Reconstr Surg. 2003; 111:2364–2369.

22. Gong X, Lu LJ. Reconstruction of severe contracture of the first web space using the reverse posterior interosseous artery flap. J Trauma. 2011; 71:1745–1749.

23. Sieg P, Dericioglu M, Hansmann C, Jacobsen HC, Trenkle T, Hakim SG. Long-term functional donor site morbidity after ulnar forearm flap harvest. Head Neck. 2012; 34:1312–1316.

24. Neuwirth M, Hubmer M, Koch H. The posterior interosseous artery flap: clinical results with special emphasis on donor site morbidity. J Plast Reconstr Aesthet Surg. 2013; 66:623–628.

25. Özalp B, Elbey H, Aydin A, Özkan T. Distally based subcutaneous veins for venous insufficiency of the reverse posterior interosseous artery flap. Microsurgery. 2016; 36:384–390.

26. Kola N, Isaraj S, Xhepa G, Belba M, Belba G. Posterior interosseous forearm flap in reconstruction of first web space. Ann Burns Fire Disasters. 2009; 22:104–106.
27. Pan ZH, Jiang PP, Wang JL. Posterior interosseous free flap for finger re-surfacing. J Plast Reconstr Aesthet Surg. 2010; 63:832–837.

28. Xu G, Lai-jin L. Coverage of skin defects in spaghetti wrist trauma: application of the reverse posterior interosseous flap and its anatomy. J Trauma. 2007; 63:402–404.

29. Jones ME, Rider MA, Hughes J, Tonkin MA. The use of a proximally based posterior interosseous adipofascial flap to prevent recurrence of synostosis of the elbow joint and forearm. J Hand Surg Eur Vol. 2007; 32:143–147.

30. Vögelin E, Langer M, Büchler U. How reliable is the posterior interosseous artery island flap? A review of 88 patients. Handchir Mikrochir Plast Chir. 2002; 34:190–194.
31. Inoue Y, Taylor GI. The angiosomes of the forearm: anatomic study and clinical implications. Plast Reconstr Surg. 1996; 98:195–210.

32. Lin SD, Lai CS, Chiu CC. Venous drainage in the reverse forearm flap. Plast Reconstr Surg. 1984; 74:508–512.

33. Puri V, Mahendru S, Rana R. Posterior interosseous artery flap, fasciosubcutaneous pedicle technique: a study of 25 cases. J Plast Reconstr Aesthet Surg. 2007; 60:1331–1337.

34. Reyad KA, Shaker AA, Elbarbary AS, Sayed MA, Elghareeb MA. The number of perforators included in reversed flow posterior interosseous artery flap: does it affect the incidence of venous congestion? Plast Reconstr Surg Glob Open. 2016; 4:e1162.
35. Acharya AM, Bhat AK, Bhaskaranand K. The reverse posterior interosseous artery flap: technical considerations in raising an easier and more reliable flap. J Hand Surg Am. 2012; 37:575–582.

36. Brunelli F, Valenti P, Dumontier C, Panciera P, Gilbert A. The posterior interosseous reverse flap: experience with 113 flaps. Ann Plast Surg. 2001; 47:25–30.

37. Akinci M, Ay S, Kamiloglu S, Erçetin O. The reverse posterior interosseous flap: a solution for flap necrosis based on a review of 87 cases. J Plast Reconstr Aesthet Surg. 2006; 59:148–152.

38. Chen HC, Cheng MH, Schneeberger AG, Cheng TJ, Wei FC, Tang YB. Posterior interosseous flap and its variations for coverage of hand wounds. J Trauma. 1998; 45:570–574.

39. Fong PL, Chew WY. Posterior interosseous artery flap: our experience and review of modifications done. Hand Surg. 2014; 19:181–187.

40. Tiengo C, Monticelli A, Bonvini S, Wassermann V, Venezia ED, Bassetto F. Critical upper limb ischemia due to brachial tourniquet in misdiagnosed thoracic outlet syndrome after carpal tunnel decompression: a case report. World J Plast Surg. 2017; 6:375–379.
41. Costa H, Smith R, McGrouther DA. Thumb reconstruction by the posterior interosseous osteocutaneous flap. Br J Plast Surg. 1988; 41:228–233.

42. Suzuki S, Iwamoto T, Koshima I. Adipofascial turnover perforator flap for dorsal hand reconstruction based on both the posterior interosseous artery and radial artery. J Hand Surg Eur Vol. 2012; 37:178–180.

43. Li KW, Song DJ, Liu J, Xie SL. Tripaddle posterior interosseous artery flap design for 3-finger defects: an evaluation of 3 surgical approaches. Ann Plast Surg. 2016; 77:406–412.
44. Sungpet A, Patradul A. The new flap based on the longitudinal fascial branches of the posterior interosseous artery. J Med Assoc Thai. 1998; 81:458–461.
45. Prasad R, Balakrishnan TM. Role of interosseous recurrent artery perforators in the posterior interosseous artery flap. J Hand Microsurg. 2015; 7:36–41.

46. Martin D, Legaillard P, Bakhach J, Hu W, Baudet J. [Reverse flow YV pedicle extension: a method of doubling the arc of rotation of a flap under certain conditions]. Ann Chir Plast Esthet. 1994; 39:403–414. French.
47. Zhuang YH, Lin J, Fu FH, Cai ZD, Huang HM, Zheng HP. The posterolateral mid-forearm perforator flap: anatomical study and clinical application. Microsurgery. 2013; 33:638–645.

48. Gao W, Yan H, Li Z, et al. The free dorsoradial forearm perforator flap: anatomical study and clinical application in finger reconstruction. Ann Plast Surg. 2011; 66:53–58.
49. Yoon CS, Noh HJ, Malzone G, Suh HS, Choi DH, Hong JP. Posterior interosseous artery perforator-free flap: treating intermediate-size hand and foot defects. J Plast Reconstr Aesthet Surg. 2014; 67:808–814.

50. Usami S, Okazaki M. Fingertip reconstruction with a posterior interosseous artery perforator flap: a minimally invasive procedure for donor and recipient sites. J Plast Reconstr Aesthet Surg. 2017; 70:166–172.

51. Vögelin E, Bignion D, Constantinescu M, Büchler U. [Revision surgery after carpal tunnel release using a posterior interosseous artery island flap]. Handchir Mikrochir Plast Chir. 2008; 40:122–127. German.
52. Pagnotta A, Taglieri E, Molayem I, Sadun R. Posterior interosseous artery distal radius graft for ulnar nonunion treatment. J Hand Surg Am. 2012; 37:2605–2610.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download