Abstract
Purpose:
Neurologic deficits after enucleation of schwannoma are not rare. To evaluate the neurologic deficits after surgical enucleation of schwannoma in the upper extremity, we performed a retrospective review of patients with surgically treated schwannoma over a 14-year period at a single institution.
Methods:
Between March 2001 and September 2014, 103 patients underwent surgical enucleation for schwannomas; 36 patients of them had lesions in the upper extremity, and 2 out of 36 patients had multiple schwannomas. Each operation was performed by a single surgeon under loupe magnification. The postoperative neurological deficits were graded as major and minor in both immediate postoperatively and at last follow-up. The major deficit was defined as anesthesia or marked hypoesthesia, motor weakness of grade 3 or less and neuropathic pain. Minor deficit was defined as mild symptoms of mild hypoesthesia, paresthesia and motor weakness of grade 4 or more.
Results:
There were 2 major (2 mixed nerve) and 12 minor (4 motor, 7 sensory, 1 mixed nerve) neurologic deficits after surgery. At the last follow-up, one major mixed neurologic deficit remained as major motor and minor sensory, and other major ones changed to mixed minor. And all minor deficits except 1 sensory deficit were recovered spontaneously.
Conclusion:
Even though high incidence rate of neurologic deficit after enucleation of schwannoma in the upper extremity (38.9%), about three fourths of them were recovered spontaneously. There were 3 permanent neurologic deficits, and one of them was major one. In some cases, surgeon cannot avoid to encounter a neurological deficit. So we recommend more delicate microscopic surgical procedure and preoperative planning and counseling. And surgery is indicated for only symptomatic lesions.
Go to : 
References
1. Donner TR, Voorhies RM, Kline DG. Neural sheath tumors of major nerves. J Neurosurg. 1994; 81:362–73.

2. Colon F, Upton J. Pediatric hand tumors: a review of 349 cases. Hand Clin. 1995; 11:223–43.
3. Strickland JW, Steichen JB. Nerve tumors of the hand and forearm. J Hand Surg Am. 1977; 2:285–91.

4. Kehoe NJ, Reid RP, Semple JC. Solitary benign peripheral-nerve tumours: review of 32 years’ experience. J Bone Joint Surg Br. 1995; 77:497–500.

5. Holdsworth BJ. Nerve tumours in the upper limb: a clinical review. J Hand Surg Br. 1985; 10:236–8.

6. Molina AR, Chatterton BD, Kalson NS, Fallowfield ME, Khandwala AR. Multiple schwannomas of the upper limb related exclusively to the ulnar nerve in a patient with segmental schwannomatosis. J Plast Reconstr Aesthet Surg. 2013; 66:e376–9.

7. Hasham S, Matteucci P, Stanley PR. Schwannomatosis: multiple schwannomas of the upper limb. J Hand Surg Br. 2006; 31:182–4.

9. Park MJ, Seo KN, Kang HJ. Neurological deficit after surgical enucleation of schwannomas of the upper limb. J Bone Joint Surg Br. 2009; 91:1482–6.

10. Mizushima H. Neurological deficits before and after surgical resection of schwannomas in the upper extremities. J Reconstr Microsurg. 2016; 32:371–7.

11. Adani R, Tarallo L, Mugnai R, Colopi S. Schwannomas of the upper extremity: analysis of 34 cases. Acta Neurochir (Wien). 2014; 156:2325–30.

12. Tang CY, Fung B, Fok M, Zhu J. Schwannoma in the upper limbs. Biomed Res Int. 2013; 2013:167196.
13. Kim SM, Seo SW, Lee JY, Sung KS. Surgical outcome of schwannomas arising from major peripheral nerves in the lower limb. Int Orthop. 2012; 36:1721–5.

14. James MA. Use of the Medical Research Council muscle strength grading system in the upper extremity. J Hand Surg Am. 2007; 32:154–6.

16. Ujigo S, Shimose S, Kubo T, Fujimori J, Ochi M. Therapeutic effect and risk factors for complications of excision in 76 patients with schwannoma. J Orthop Sci. 2014; 19:150–5.

17. Levi AD, Ross AL, Cuartas E, Qadir R, Temple HT. The surgical management of symptomatic peripheral nerve sheath tumors. Neurosurgery. 2010; 66:833–40.

18. Forthman CL, Blazar PE. Nerve tumors of the hand and upper extremity. Hand Clin. 2004; 20:233–42.

19. Sawada T, Sano M, Ogihara H, Omura T, Miura K, Nagano A. The relationship between pre-operative symptoms, operative findings and postoperative complications in schwannomas. J Hand Surg Br. 2006; 31:629–34.

20. Artico M, Cervoni L, Wierzbicki V, D’Andrea V, Nucci F. Benign neural sheath tumours of major nerves: characteristics in 119 surgical cases. Acta Neurochir (Wien). 1997; 139:1108–16.

21. Oberle J, Kahamba J, Richter HP. Peripheral nerve schwannomas: an analysis of 16 patients. Acta Neurochir (Wien). 1997; 139:949–53.
22. Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007; 89:382–7.
Go to : 
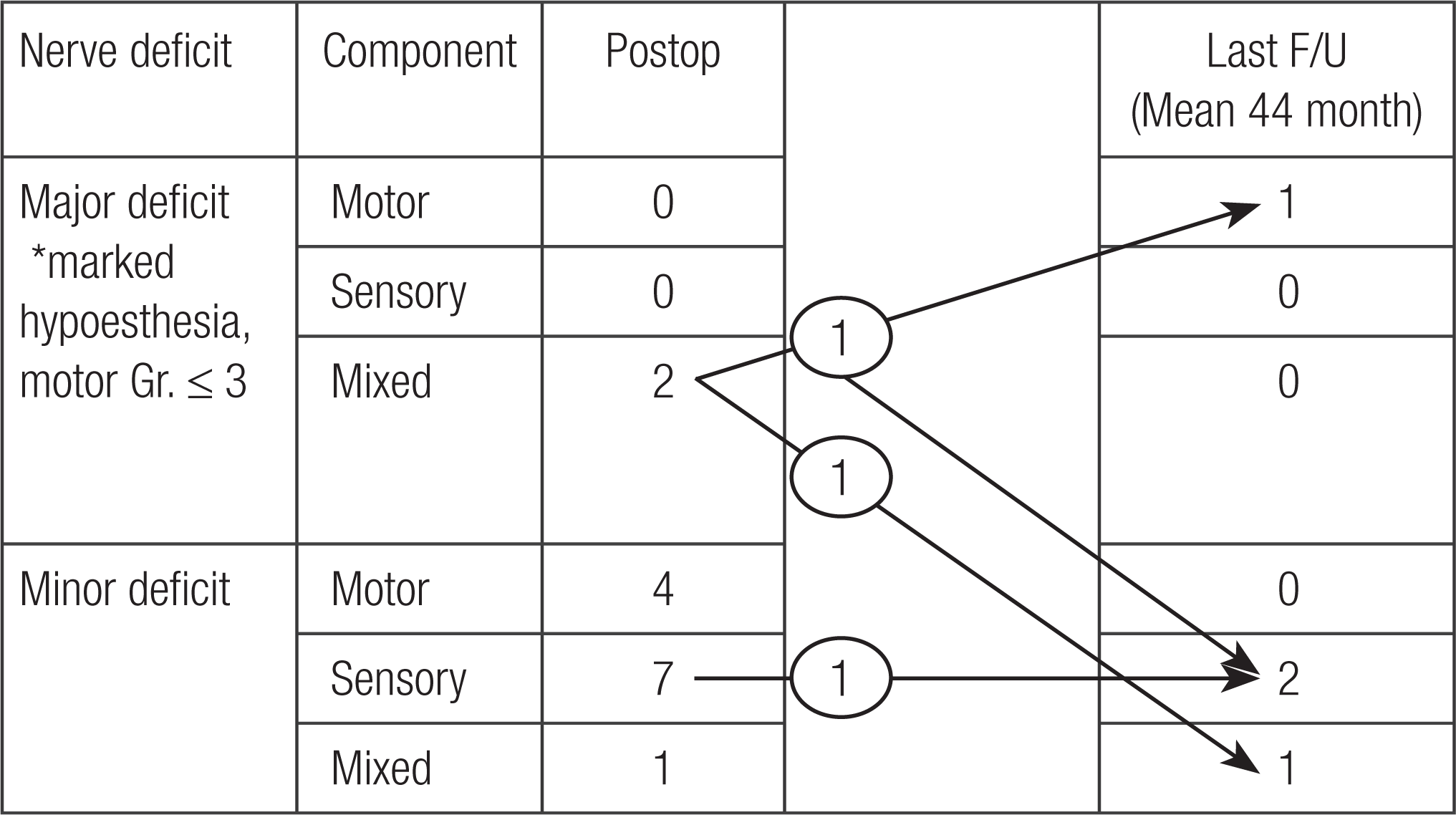 | Fig. 1.Neurologic deficit. At the last follow-up examination, among the patients who have had neurologic deficits (major deficit: 1 case, minor deficit: 2 cases), mixed neurologic deficit case ultimately determined to have major motor deficit and minor sensory deficit. Therefore 1 major deficit and 3 minor deficits left finally. Postop, postoperative; Gr., grade. * Definition of major deficit. |
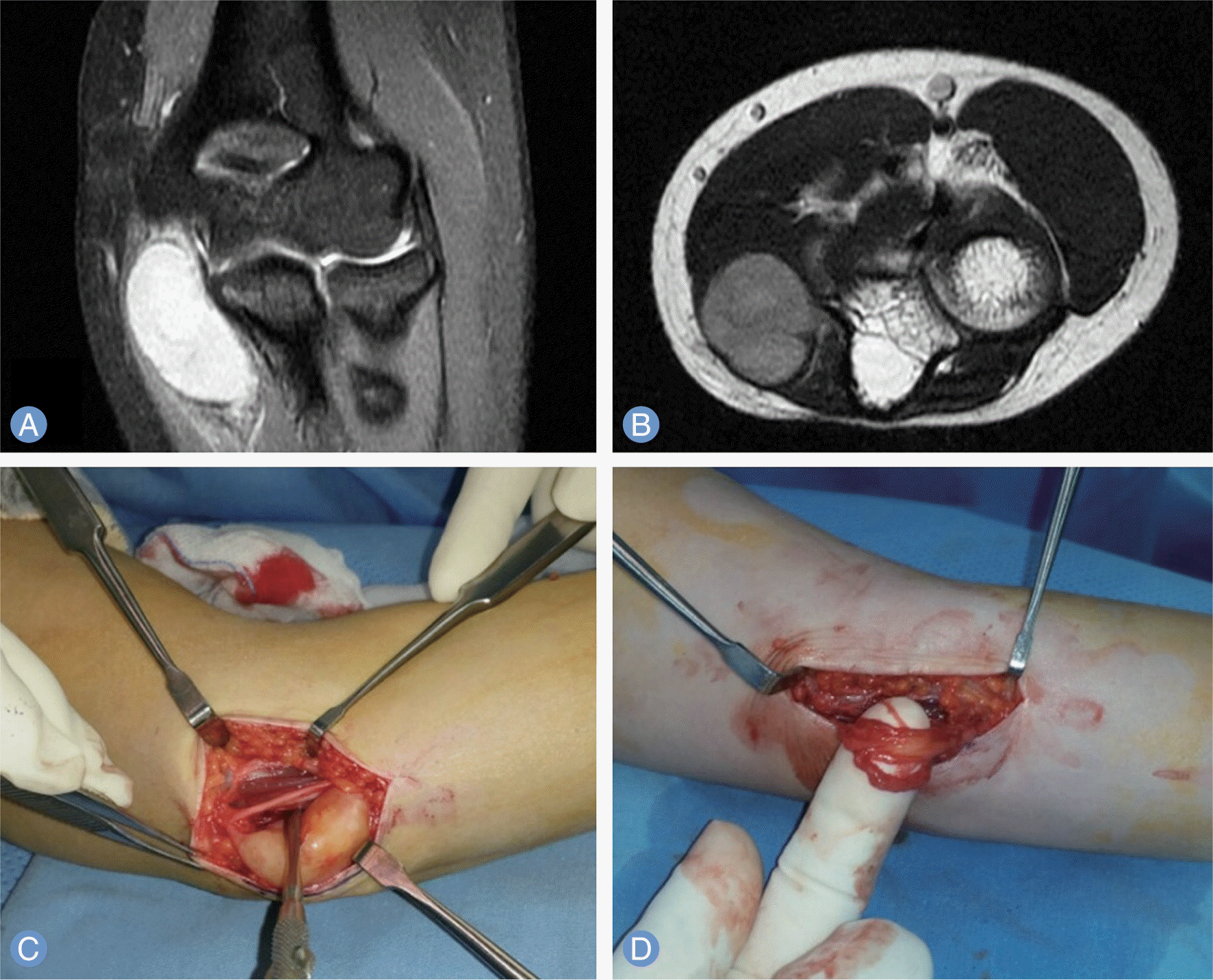 | Fig. 2.Magnetic resonance imaging of 31-year-old female patients shows schwannoma near cubital tunnel of elbow (A, B). One nerve fascicle was entering the tumor, and we could not dissect the fascicle from the tumor. So we left some of the tumor untouched, to avoid nerve injury (C, D). |
Table 1.
Locations of the schwannomas and the nerves involved
| Nerve | Axilla | Arm | Elbow | Forearm | Wrist & hand |
|---|---|---|---|---|---|
| Ulnar | 4 | 4 | 2 | - | 2 |
| Median | 1 | 2 | 1 | 1 | 4 |
| Radial | - | 5 | 1 (SRN) | 3 (SRN, PIN, RN) | 1 (SRN) |
| Musculocut | - | - | 1 | - | - |
| Digital | - | - | - | - | 6* |
| Total | 5 | 11 | 5 | 4 | 13 |
| SRN, superficial radial nerve; PIN, posterior interosseous nerve; RN, radial nerve. | |||||
Table 2.
Analysis for risk factors of postoperative neurologic deficit in schwannoma patients
| Characteristic | All (n=36) | Neurologic deficit (+) (n=14) | Neurologic deficit (–) (n=22) | p-value |
|---|---|---|---|---|
| Tinel sign | 0.29 | |||
| Positive | 24 | 11 | 13 | |
| Negative | 12 | 3 | 9 | |
| Age (yr) | 0.45 | |||
| 20–40 | 10 | 5 | 5 | |
| 40–60 | 17 | 6 | 11 | |
| >60 | 9 | 3 | 6 | |
| Gender | 1.00 | |||
| Male | 12 | 5 | 7 | |
| Female | 24 | 9 | 15 | |
| Size (cm) | 0.49 | |||
| <3 | 18 | 6 | 12 | |
| >3 | 18 | 8 | 10 | |
| Location | 0.30 | |||
| Above elbow | 21 | 10 | 11 | |
| Below elbow | 15 | 4 | 11 | |
| Importance of nerve | 0.05* | |||
| Major nerve | 26 | 13 | 13 | |
| Minor nerve | 10 | 1 | 9 | |
| No. of mass | 0.51 | |||
| Solitary | 34 | 14 | 20 | |
| Multiple | 2 | 0 | 2 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download