Abstract
Purpose
A barbed suture used in flexor tenorrhaphy can maintain prolonged strength despite absorption of the suture material and allows knotless repair with tendon-barb adherence along the suture's entire length. The purpose of this study was to evaluate the strength of the tendon and its histologic analysis after tenorrhaphy using barbed sutures.
Methods
Forty-two New Zealand rabbits were used in this study and were divided into experimental and control groups. In the experimental group, knotless repair of the tendons was performed using absorbable barbed sutures. In the control group, a 4-stranded double-modified Kessler tenorrhaphy was performed using non-absorbable monofilament sutures. The force to failure for each tendon was measured immediately after tendon repair and at 1 week, 4 weeks, and 8 weeks after the repair. Microscopic analysis of the tendons was performed at 1 week, 4 weeks, and 8 weeks after their repair.
Results
Eight weeks after tendon repair, the force to failure value of the rabbits in the experimental group (144.02±10.21 N) was significantly higher than that of the rabbits in the control group (125.26±8.75 N) (p=0.032). The difference in the value during the periods was not statistically significant. Histologic findings showed increased foreign body reaction in the tendons of the experimental group and sustained inflammation in those of the control group.
Figures and Tables
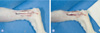 | Fig. 2Intraoperative photographs. (A) Tenotomy after careful dissection of the tendo calcaneus. (B) Picture taken after tendon repair. |
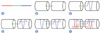 | Fig. 4Illustration of barbed suture tenorrhaphy. (A) Schematic of bidirectional barbed suture with central unbarbed segment and opposing barbed segments. (B) Central segment aligned in the gap between cut tendon ends. (C) First needle advanced through tendon, parallel to the direction of the fibrils, for a distance of 0.5 cm, and secured with two transverse passes perpendicular to the direction of the tendon fibrils. (D) Needle advanced parallel to the fibrils to cross injury site. (E) Two additional transverse passes made to anchor the suture. (F) Process repeated with second needle in opposite end of tendon to complete symmetric knotless three-strand repair. |
 | Fig. 5Eight weeks after repair. (A) Gross picture of a harvested tendon 8 weeks after its repair with Quill sutures. Fibrous adhesion, which were seen at the 4th week decreased. (B) Tendon harvested 8 weeks after its repair using Prolene shows decreased fibrotic material but seems slightly more severe than the Quill group of the same period. |
 | Fig. 6Force until failure. All values depicted are means with standard deviations. Significant differences are indicated by asterix (*p<0.05). |
 | Fig. 7Optical microscopy findings 1 week after tendon repair. (A) Experimental group. Around the suture hole, fibroblast proliferation is associated with inflammatory cell infiltration. Focal edematous changes make gaps between new fibrotic layer and mature collagen layer. Inflammation degree is (++). H&E, ×200. (B) Control group. Microscopic changes are similar to those of experimental group. Edematous changes are more severe compared to the experimental group. Inflammation grade is (++). H&E, ×200. |
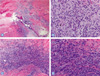 | Fig. 8Optical microscopy findings 4 weeks after tendon repair. (A) Experimental group, newly formed collagen bundles are seen adjacent to the inflammatory cells. H&E, ×200. (B) Experimental group, Amorphous hyaline materials (*) are surrounded by mononulcear cells and they together are forming granuloma-like lesions. Overall inflammation is (++) to (+++). H&E, ×400. (C) Control group. Inflammatory cell infiltration in the area of fibrolastic proliferation is more severe than that of the 1st week. The orientation of the collagen bundles is not mature. H&E, ×200. (D) Control group has no signs of amorphous hyaline materials. The overall inflammation was (+++). H&E, ×400. |
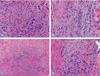 | Fig. 9Optical microscopy findings 8 weeks after tendon repair. (A) Experimental group. Regenerating area is composed of fibroblasts with few inflammatory cells. There are also very small undigested pieces of amorphous material (*). H&E, ×400. (B) Experimental group. Most collagen bundles are similar to normal in terms of arrangement. But still fibroblasts and capillaries lined with plump endothelial cells are present. Overall inflammation is (+). H&E, ×400. (C) Regenerating area shows proliferated capillaries with somewhat irregular budding and still scattered fibroblasts are present. H&E, ×200. (D) Control group has deposition of relatively mature pattern fibroblast and collagen (arrows). The overall inflammation was (+) to (++). H&E, ×400. |
References
1. Strickland JW. Flexor tendon surgery. Part 1: primary flexor tendon repair. J Hand Surg Br. 1989. 14:261–272.
2. Silfverskiold KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg Am. 1994. 19:53–60.
3. Thurman RT, Trumble TE, Hanel DP, Tencer AF, Kiser PK. Two-, four-, and six-strand zone II flexor tendon repairs: an in situ biomechanical comparison using a cadaver model. J Hand Surg Am. 1998. 23:261–265.
4. Zaruby J, Gingras K, Taylor J, Maul D. An in vivo comparison of barbed suture devices and conventional monofilament sutures for cosmetic skin closure: biomechanical wound strength and histology. Aesthet Surg J. 2011. 31:232–240.

5. Parikh PM, Davison SP, Higgins JP. Barbed suture tenorrhaphy: an ex vivo biomechanical analysis. Plast Reconstr Surg. 2009. 124:1551–1558.

7. Schuind F, Garcia-Elias M, Cooney WP 3rd, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg Am. 1992. 17:291–298.

8. Lawrence TM, Davis TR. A biomechanical analysis of suture materials and their influence on a four-strand flexor tendon repair. J Hand Surg Am. 2005. 30:836–841.

9. Villa MT, White LE, Alam M, Yoo SS, Walton RL. Barbed sutures: a review of the literature. Plast Reconstr Surg. 2008. 121:102e–108e.

10. Rashid RM, Sartori M, White LE, Villa MT, Yoo SS, Alam M. Breaking strength of barbed polypropylene sutures: rater-blinded, controlled comparison with nonbarbed sutures of various calibers. Arch Dermatol. 2007. 143:869–872.
11. Mashadi ZB, Amis AA. Variation of holding strength of synthetic absorbable flexor tendon sutures with time. J Hand Surg Br. 1992. 17:278–281.

12. Enwemeka CS. Functional loading augments the initial tensile strength and energy absorption capacity of regenerating rabbit Achilles tendons. Am J Phys Med Rehabil. 1992. 71:31–38.

14. Strickland JW. Flexor Tendon Injuries: I. Foundations of Treatment. J Am Acad Orthop Surg. 1995. 3:44–54.

15. Yildirim Y, Kara H, Cabukoglu C, Esemenli T. Suture holding capacity of the Achilles tendon during the healing period: an in vivo experimental study in rabbits. Foot Ankle Int. 2006. 27:121–124.

16. Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg Am. 2000. 25:214–235.

17. Pruitt DL, Manske PR, Fink B. Cyclic stress analysis of flexor tendon repair. J Hand Surg Am. 1991. 16:701–707.

18. Williams RJ, Amis AA. A new type of flexor tendon repair. Biomechanical evaluation by cyclic loading, ultimate strength and assessment of pulley friction in vitro. J Hand Surg Br. 1995. 20:578–583.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download