Abstract
Among different graft materials for craniofacial reconstruction, calcium phosphate cements have the advantages of alloplastic grafts and wide use. The authors report a case of foreign body reaction following frontal reconstruction with JectOS (an injectable calcium orthophosphate cement; Kasios) and reviewed the literature on complications of this material after craniofacial reconstruction from 2002 to 2017. Complications were categorized into two groups: immunologic reactions (consisting of seroma collection, chronic sinus mucosa swelling, and foreign body reaction) and non-immune events (infection, fragmentation, and ejection). It is wise to use calcium phosphate-based material only in selected cases with small defects, and long-term follow-up is needed to observe their consequences.
Among several bone substitutes that are used for craniofacial reconstruction, the known properties of alloplastic materials including no donor site morbidity, less operation time and complexity, and less probability of cross infection, transcend the disadvantages of autograft, allograft, and xenograft123. Calcium phosphate-based materials are analogous to inorganic bone matrix4. Innovations in their cement form overcome the shortage of their ceramic form; because of osteoconductivity, good moldability, and structural stability, they are widely used for craniofacial defects5678. Although several studies indicate the biocompatibility of calcium phosphate-based materials91011, there are reports of foreign body reaction and seroma collection after craniofacial reconstruction using different cements such as Norian CRS (Synthes-Stratec, Oberdorf, Switzerland), Mimix (Walter Lorenz Surgical, Jacksonville, FL, USA), and Bone Source (Stryker Leibinger, Freiburg, Germany)1213141516. We report a patient who showed foreign body reaction following use of JectOS, an injectable calcium orthophosphate cement (Kasios, Launaguet, France), for reconstruction of a frontal bone defect. We also reviewed literature from 2002 to 2017 that reported complications of calcium phosphate cements after craniofacial reconstruction121314151617181920212223242526.(Table 1)
Search terms of craniofacial, frontal, complication, and calcium phosphate cement were submitted to ScienceDirect, PubMed, and Google Scholar databases. Only English articles that reported complications after craniofacial reconstruction with this material were included.
To the best of our knowledge, no adverse effects of JectOS were published following craniofacial application.
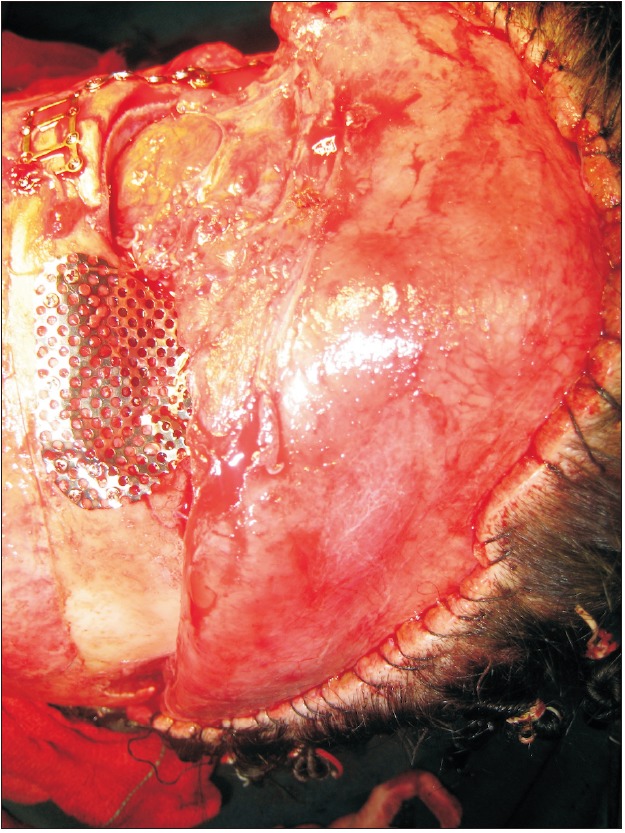
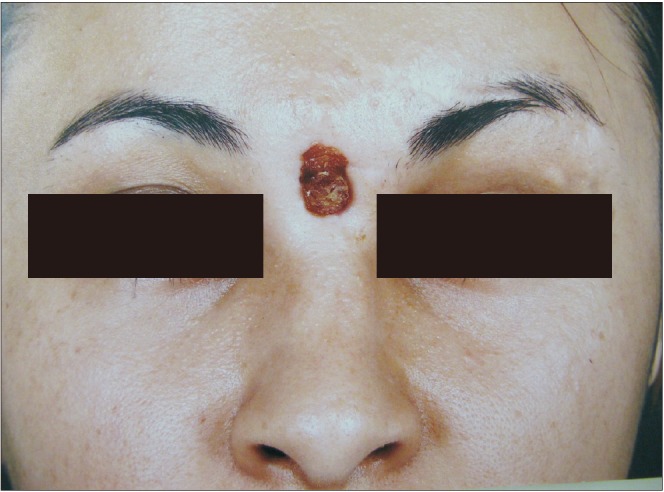
A 28-year-old woman was referred to the Oral and Maxillofacial unit of Taleghani Hospital (Tehran, Iran) in 2010 because of multiple facial fractures. Physical examination and radiographic study revealed bilateral naso-orbito-ethmoid and zygomaticomaxillary complex fractures and fracture of anterior and posterior tables of the frontal sinus with displacement. The naso-frontal duct was intact, and there was no cerebrospinal fluid outflow. The patient underwent open reduction and rigid internal fixation of the complex fractures and reconstruction of the frontal anterior table with titanium mesh and JectOS.(Fig. 1, 2) Four months later, the patient presented with a midfrontal fistula.(Fig. 3) Exudate culture did not show any bacterial growth, and the patient underwent debridement and fistulectomy. The pathological study showed granulation tissue and giant cells, confirming a foreign body reaction. Nine months later, because the frontal drainage did not stop and several outpatient irrigation and debridement procedures were not successful, debridement and removal of the reconstructive titanium mesh and JectOS were performed, and a calvarial autograft was used to reconstruct the frontal depressed defect. The postoperation course and 6-year follow-up were uneventful.(Fig. 4)
From a cosmetic stand point and because of little stress on the craniofacial structure, use of calcium phosphate biomaterials for craniofacial reconstruction is desirable. Currently, two major groups of calcium phosphate cements are available: apatite cements with poorly crystalline hydroxyapatite (HA) and calcium-deficient HA (CDHA), and dicalcium phosphate dihydrate (DCPD) cements, also called brushite, such as B-tricalcium phosphate (B-TCP)4.
Complications after calcium phosphate biomaterial application are divided into two categories: immunologic reactions27 and non-immunologic events such as infection1517, fragmentation, ejection, and migration1819.
According to the literature, proximity of the incision line to the surgical site, wound tension that results in wound dehiscence, previous radiation therapy, and minor trauma at the site of surgery are probable reasons for infection after the use of these materials212202124.
Some research reported fragmentation and ejection of calcium phosphate cements contacting the dura and proposed the use of a protective mesh under cement to prevent dura pulse transmission1922.
Zins et al.2528 reported a high rate of complications after reconstruction of a large, full-thickness cranial defect with Bone Source and Norian CRS and suggested the use of autogenous graft for reconstruction of these patients.
Immunologic host reactions following implantation of biomaterials include blood-material interactions and acute or chronic inflammation. Although early resolution of the inflammatory response is expected after application of a biocompatible material, the formation of granulation tissue after chronic inflammation results in a foreign body reaction and fibrous capsule formation2729.
Development of tissue reactions, like persistent swelling and seroma collection or chronic drain fistula, after cranio-facial application of HA or calcium phosphate cements results in surgeons not using these biomaterials in contact with the sinus mucosa12131523. This reaction was observed among all ages, and no difference between genders was reported23. According to these reports, the use of Bone Source, Mimix, or Norian CRS to reconstruct the frontal deepening is not desired, especially if the sinus mucosa is exposed.
JectOS is a calcium orthophosphate cement made up of 55% DCPD and 45% TCP. Uygur et al.30 reported a case of soft tissue necrosis around a lateral malleolar region following the filling of a calcaneus bone cyst with JectOS. On the second and third days postoperative, local pain, burning sensation, erythema, and serous fluid leakage in the injection region resulted in skin and soft tissue necrosis with no evidence of deep infection.
In our experience with JectOS, host reaction symptoms were evident after four months. Multiple outpatient procedures did not stop the chronic discharge; therefore, after nine months, the patient underwent complete graft removal, and no evidence of local infection or fragmentation was observed.
Therefore, the rate of postsurgical failure, including infection, following biomaterial usage, is high due to inadequate blood supply and infection control disturbance. Most of the research describing biomaterial complications report infection as the predominant side effect; however, in this case report, the patient did not suffer from infection. From a management perspective and time to occurrence, this case had more serious complications than other reports.
According to the literature, application of calcium phosphate biomaterials consisting of JectOS on fractured frontal bone in contact with sinus membrane and disrupted blood supply can result in foreign body reaction and infection. Therefore, long-term follow-up after biomaterial application is suggested.
Notes
Authors' Contributions: F.P. participated in data collection and helped to draft the manuscript. N.L. participated in the literature analysis. F.L. participated in the study design and coordination and manuscript writing. All authors read and approved the final manuscript.
References
1. Neumann A, Kevenhoerster K. Biomaterials for craniofacial reconstruction. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2009; 8:Doc08. PMID: 22073101.
2. Rodriguez ED, Stanwix MG, Nam AJ, St Hilaire H, Simmons OP, Christy MR, et al. Twenty-six-year experience treating frontal sinus fractures: a novel algorithm based on anatomical fracture pattern and failure of conventional techniques. Plast Reconstr Surg. 2008; 122:1850–1866. PMID: 19050539.

3. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005; 36(Suppl 3):S20–S27. PMID: 16188545.

4. Dorozhkin SV. Self-setting calcium orthophosphate formulations: cements, concretes, pastes and putties. Int J Mat Chem. 2011; 1:1–48.

5. Neamat A, Gawish A, Gamal-Eldeen AM. Beta-tricalcium phosphate promotes cell proliferation, osteogenesis and bone regeneration in intrabony defects in dogs. Arch Oral Biol. 2009; 54:1083–1090. PMID: 19828137.
6. Ooms EM, Wolke JG, van de Heuvel MT, Jeschke B, Jansen JA. Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials. 2003; 24:989–1000. PMID: 12504521.

7. Maier W. Biomaterials in skull base surgery. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2009; 8:Doc07. PMID: 22073100.
8. Obert L, Lepage D, Gasse N, Rochet S, Garbuio P. Extra-articular distal radius malunion: the phosphate cement alternative. Orthop Traumatol Surg Res. 2010; 96:574–578. PMID: 20634164.

9. Nilsson M, Wang JS, Wielanek L, Tanner KE, Lidgren L. Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg Br. 2004; 86:120–125. PMID: 14765879.

10. Chun BD, Kim SW, Lee ST, Kim TH, Lee JH, Kim GC, et al. Interaction between odontoblast and bio-calcium phosphate cement reinforced with chitosan. J Korean Assoc Oral Maxillofac Surg. 2011; 37:415–420.

11. Hönig JF, Merten HA, Nitsch A, Verheggen R. Contouring of cranial vault irregularities with hydroxyapatite cement: a clinical and experimental investigation. J Craniofac Surg. 2005; 16:457–460. PMID: 15915115.

12. Matic D, Phillips JH. A contraindication for the use of hydroxyapatite cement in the pediatric population. Plast Reconstr Surg. 2002; 110:1–5. PMID: 12087221.

13. Magee WP Jr, Ajkay N, Freda N, Rosenblum RS. Use of fast-setting hydroxyapatite cement for secondary craniofacial contouring. Plast Reconstr Surg. 2004; 114:289–297. PMID: 15277791.

14. Verret DJ, Ducic Y, Oxford L, Smith J. Hydroxyapatite cement in craniofacial reconstruction. Otolaryngol Head Neck Surg. 2005; 133:897–899. PMID: 16360510.

15. Mathur KK, Tatum SA, Kellman RM. Carbonated apatite and hydroxyapatite in craniofacial reconstruction. Arch Facial Plast Surg. 2003; 5:379–383. PMID: 12975134.

16. Eppley BL, Hollier L, Stal S. Hydroxyapatite cranioplasty: 2. Clinical experience with a new quick-setting material. J Craniofac Surg. 2003; 14:209–214. PMID: 12621292.

17. Durham SR, McComb JG, Levy ML. Correction of large (>25 cm(2)) cranial defects with “reinforced” hydroxyapatite cement: technique and complications. Neurosurgery. 2003; 52:842–845. discussion 845. PMID: 12657179.

18. Kerr RG, Hearst MJ, Samy RN, van Loveren HR, Tew JM Jr, Pensak ML, et al. Delayed extrusion of hydroxyapatite cement after transpetrosal reconstruction. Neurosurgery. 2009; 64:527–531. discussion 531-2. PMID: 19240615.

19. David L, Argenta L, Fisher D. Hydroxyapatite cement in pediatric craniofacial reconstruction. J Craniofac Surg. 2005; 16:129–133. PMID: 15699660.

20. Gómez E, Martín M, Arias J, Carceller F. Clinical applications of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children: our experience at Hospital La Paz since 2001. J Oral Maxillofac Surg. 2005; 63:8–14. PMID: 15635550.

21. Gosain AK, Chim H, Arneja JS. Application-specific selection of biomaterials for pediatric craniofacial reconstruction: developing a rational approach to guide clinical use. Plast Reconstr Surg. 2009; 123:319–330. PMID: 19116568.

22. Greenberg BM, Schneider SJ. Alloplastic reconstruction of large cranio-orbital defects: a comparative evaluation. Ann Plast Surg. 2005; 55:43–51. discussion 51. PMID: 15985790.
23. Gilardino MS, Cabiling DS, Bartlett SP. Long-term follow-up experience with carbonated calcium phosphate cement (Norian) for cranioplasty in children and adults. Plast Reconstr Surg. 2009; 123:983–994. PMID: 19319064.

24. Singh KA, Burstein FD, Williams JK. Use of hydroxyapatite cement in pediatric craniofacial reconstructive surgery: strategies for avoiding complications. J Craniofac Surg. 2010; 21:1130–1135. PMID: 20613591.
25. Zins JE, Moreira-Gonzalez A, Papay FA. Use of calcium-based bone cements in the repair of large, full-thickness cranial defects: a caution. Plast Reconstr Surg. 2007; 120:1332–1342. PMID: 17898609.

26. Baker SB, Weinzweig J, Kirschner RE, Bartlett SP. Applications of a new carbonated calcium phosphate bone cement: early experience in pediatric and adult craniofacial reconstruction. Plast Reconstr Surg. 2002; 109:1789–1796. PMID: 11994575.

27. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008; 20:86–100. PMID: 18162407.

28. Zins JE, Langevin CJ, Nasir S. Controversies in skull reconstruction. J Craniofac Surg. 2010; 21:1755–1760. PMID: 21119415.

29. Rokn AR, Khodadoostan MA, Reza Rasouli Ghahroudi AA, Motahhary P, Kharrazi Fard MJ, Bruyn HD, et al. Bone formation with two types of grafting materials: a histologic and histomorphometric study. Open Dent J. 2011; 5:96–104. PMID: 21760862.

30. Uygur F, Ulkür E, Pehlivan O, Celiköz B. Soft tissue necrosis following using calcium phosphate cement in calcaneal bone cyst: case report. Arch Orthop Trauma Surg. 2008; 128:1397–1401. PMID: 18058113.

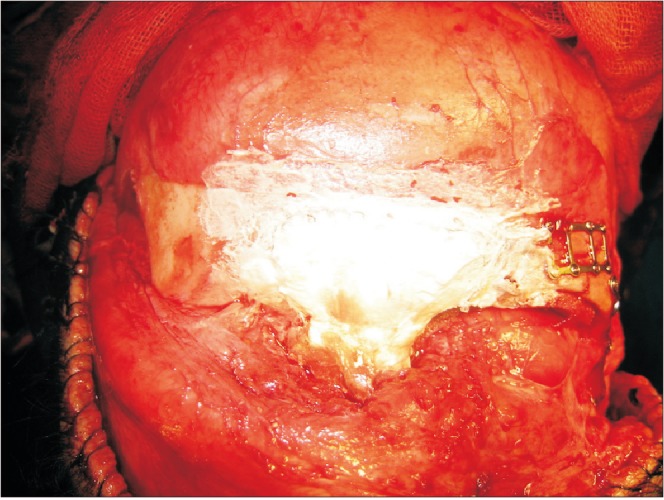
Fig. 4
Postoperation course after debridement and removal of the reconstructive titanium mesh and JectOS (Kasios).
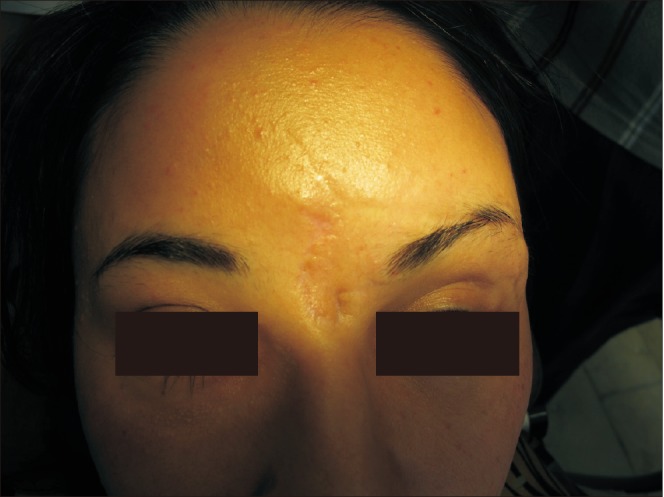
Table 1
Frequency of reported complications after craniofacial reconstruction with different calcium-phosphate cements from 2002 to 2017
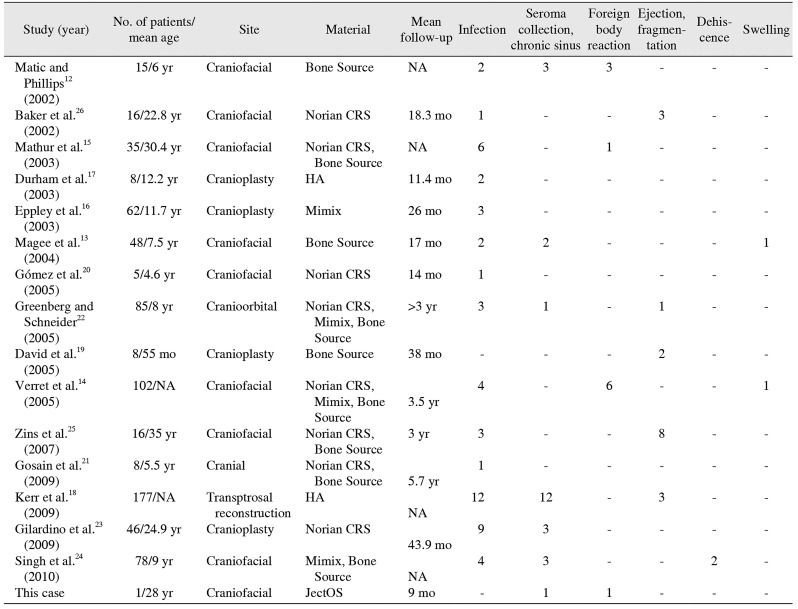
| Study (year) | No. of patients/mean age | Site | Material | Mean follow-up | Infection | Seroma collection, chronic sinus | Foreign body reaction | Ejection, fragmentation | Dehiscence | Swelling |
|---|---|---|---|---|---|---|---|---|---|---|
| Matic and Phillips12 (2002) | 15/6 yr | Craniofacial | Bone Source | NA | 2 | 3 | 3 | - | - | - |
| Baker et al.26 (2002) | 16/22.8 yr | Craniofacial | Norian CRS | 18.3 mo | 1 | - | - | 3 | - | - |
| Mathur et al.15 (2003) | 35/30.4 yr | Craniofacial | Norian CRS, Bone Source | NA | 6 | - | 1 | - | - | - |
| Durham et al.17 (2003) | 8/12.2 yr | Cranioplasty | HA | 11.4 mo | 2 | - | - | - | - | - |
| Eppley et al.16 (2003) | 62/11.7 yr | Cranioplasty | Mimix | 26 mo | 3 | - | - | - | - | - |
| Magee et al.13 (2004) | 48/7.5 yr | Craniofacial | Bone Source | 17 mo | 2 | 2 | - | - | - | 1 |
| Gómez et al.20 (2005) | 5/4.6 yr | Craniofacial | Norian CRS | 14 mo | 1 | - | - | - | - | - |
| Greenberg and Schneider22 (2005) | 85/8 yr | Cranioorbital | Norian CRS, Mimix, Bone Source | >3 yr | 3 | 1 | - | 1 | - | - |
| David et al.19 (2005) | 8/55 mo | Cranioplasty | Bone Source | 38 mo | - | - | - | 2 | - | - |
| Verret et al.14 (2005) | 102/NA | Craniofacial | Norian CRS, Mimix, Bone Source | 3.5 yr | 4 | - | 6 | - | - | 1 |
| Zins et al.25 (2007) | 16/35 yr | Craniofacial | Norian CRS, Bone Source | 3 yr | 3 | - | - | 8 | - | - |
| Gosain et al.21 (2009) | 8/5.5 yr | Cranial | Norian CRS, Bone Source | 5.7 yr | 1 | - | - | - | - | - |
| Kerr et al.18 (2009) | 177/NA | Transptrosal reconstruction | HA | NA | 12 | 12 | - | 3 | - | - |
| Gilardino et al.23 (2009) | 46/24.9 yr | Cranioplasty | Norian CRS | 43.9 mo | 9 | 3 | - | - | - | - |
| Singh et al.24 (2010) | 78/9 yr | Craniofacial | Mimix, Bone Source | NA | 4 | 3 | - | - | 2 | - |
| This case | 1/28 yr | Craniofacial | JectOS | 9 mo | - | 1 | 1 | - | - | - |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download