Abstract
Meningioma is relatively common, benign, and extra-axial tumor accounting for about 20% of primary brain and spinal cord tumors. The World Health Organization (WHO) classified these tumors into Grade I (benign), Grade II (atypical), and Grade III (anaplastic) meningioma. Grade I meningioma which is slowly growing tumor and have some rare subtypes. Among them, metaplastic subtype is defined as a tumor containing focal or widespread mesenchymal components including osseous, cartilaginous, lipomatous, myxoid or xanthomatous tissue, singly or in combinations. We report a rare metaplastic meningioma overspreading nearly whole cerebral convexity from main extra-axial tumor bulk in the parietal lobe.
Meningiomas are usually benign tumors derived from meningothelial cells and they can show various histo-pathological features. The World Health Organization (WHO) classified these tumors into Grade I (benign), Grade II (atypical), and Grade III (anaplastic) meningiomas and it divided grade I meningioma into 9 subtypes : meningothelial, fibrous, transitional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte rich, and metaplastic [1]. Metaplastic meningioma is the rarest subtype, and defined as a tumor containing focal or widespread mesenchymal components including osseous, cartilaginous, lipomatous, myxoid or xanthomatous tissue, singly or in combinations [1]. We present a rare metaplastic meningioma overlying nearly whole cerebral convexity from main extra-axial tumor bulk in the parietal lobe just like flat subdural mass.
A 28-year-old male presented with intermittent right upper extremity weakness. Motor weakness is a major cause of brain tumors. So we asked him prior consent to study his disease. Brain CT showed a large low density lesion with mass effect in the left parietal lobe. A well demarcated mass with multiple calcifications had broad dural base suggesting extra-axial tumor (Fig. 1). Brain MRI showed that the tumor mass was composed of two parts. One located in the parietal lobe showed low-signal intensity on T1-weighted image (WI) and highsignal intensity on T2-WI. Some focal heterogeneous signal intensities within the tumor mass were also seen. The other overspreading cerebral convexity in the subdural space showed isosignal intensity on T1-WI and high-signal intensity on T2-WI. Inhomogeneous and homogeneous enhancements were seen in bulky parietal mass and flat subdural mass respectively (Fig. 2). On the cerebral angiograms, this tumor was only supplied by left middle meningeal artery and was embolized successfully (Fig. 3). A large craniotomy was performed. Bulky and soft tumor mass with multiple small cysts and calcifications was observed in the parasagittal space of left parietal lobe. Flat and rubbery tumor mass in the subdural space was overspreading the nearly whole cerebral convexity. These tumors were totally extra-axial and excised completely. Parasagittal dura attaching the bulky parietal mass was considered as tumor origin site and coagulated (Fig. 4). Postoperatively, neurological symptoms were recovered. These specimens were compatible with metaplastic meningioma containing bone, fat, and xanthomatous tissues (Fig. 5).
Meningiomas are relatively common, and usually benign tumors in extra-axial location derived from meningothelial cells which have histological diversity. By WHO classification, metaplastic meningioma is recognized as grade I tumor which is characterized by various mesenchymal tissues including bone, cartilage, fat, and xanthomatous tissues. The term metaplastic has been used because their transformed neoplastic cells demonstrate the full histological characteristics of the cells they mimic. The majority of metaplastic meningioma seem to be osseous subtype [2]. However, their biological behavior is poorly understood because of limited number of reported cases [3]. Their immunohistochemical findings are similar to the other meningioma subtypes, and show negative immunoreactivity for glial fibrillary acidic protein (GFAP), and positive immunoreactivity for vimentin and epithelial membrane antigen (EMA) which reflect their dual mesenchymal and epithelial properties. A few metaplastic tumors showing positive immunoreactivity for smooth muscle actin (SMA) suggest muscular differentiation. In a meningioma, lipomatous component is considered as an advanced lipidization of neoplastic meningothelial cells rather than true metaplastic transformation into mature fat tissue [4]. Although there are no known retrospective cohort studies with long term follow up with regard to the natural course, proper treatment, and its anticipated prognosis, they are believed to have good prognosis with little recurrences [3]. The current literatures indicates that metaplastic menigioma grows like other grade I meningiomas with similar recurrence rates [5]. But, there are few cases of metaplastic meningioma was reported: 12 cases of osteoblastic meningioma, one case of cartilaginous meningioma, 4 cases of xanthomatous meninigioma and 9 cases of lipomatous meningioma. Our case is mixed with xanthomatous and lipomatous type. Furthermore, there is a case about osteochondroma which is a rare variant of non-meningothelial mesenchymal tumors arising from the meninges [6]. In these reasons, non-meningothelial mesenchymal tumor simulating metaplastic meningioma should also be included in differential diagnosis [6].
We report a rare metaplastic meningioma overlying nearly whole cerebral convexity and review relevant literatures. Metaplastic meningiomas may follow a long standing tumor development. There is no therapeutic protocol, so we must be careful to manage of that tumors and keep in mind to differential diagnosis of other meningiomas.
Figures and Tables
Fig. 1
Brain CT shows about 6.7×4.5 cm sized, low-density, broad dural based, and inhomogeneous enhancing mass with multiple calcifications in the left parietal region which is suggesting extra-axial tumor. Definite subdural lesion is not seen on the brain CT.
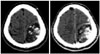
Fig. 2
Brain MRI shows extra-axial inhomogeneous enhancing tumor in the left fronto-parietal region. Bulky parietal mass shows low-signal intensity and the other flat subdural mass overspreading cerebral convexity shows iso-signal intensity on T1-weighted image (WI) (A). Inhomogeneous and homogeneous enhancements are seen in bulky parietal mass and flat subdural mass respectively (B). High-signal intensity in bulky parietal and flat subdural mass are seen on T2-WI (C). On fluid attenuated inversion recovery image, iso-signal intensity in the bulky parietal mass and high-signal intensity in the flat subdural mass are seen (D).
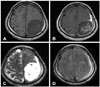
Fig. 4
Operative findings. Bulky and soft tumor mass with multiple small cysts and calcifications are seen in the parasagittal space of left parietal lobe. Flat and rubbery tumor mass in the subdural space is overspreading the nearly whole cerebral convexity. These tumors are totally extra-axial. And the tumors were not infiltrated to the brain parenchyme.
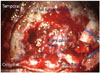
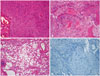
Fig. 5
Pathologic findings. A: The tumor composed of plump, elongated cells with meningothelial features in syncytial arrangement. The nuclei are round to ovoid harboring fine chromatin and inconspicuous nucleoli. There is no evidence of mitosis or necrosis (hematoxylin-eosin staining, ×10). B: The areas of chicken-wire-like calcification and ossification are seen in several areas (hematoxylin-eosin staining, ×40). C: Lipomatous metaplasia and xanthomatous change are present (hematoxylin-eosin staining, ×100). D: The tumor cells show a low Ki-67 labelling index with about 1% (immunohistochemistry staining, ×40).
References
1. Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningiomas. In : Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. World Health Organization classification of tumours of the central nervous system. Lyon: IARC Press;2007. p. 164–172.
2. Huang J, Petersson F. Intracerebral metaplastic meningioma with prominent ossification and extensive calcification. Rare Tumors. 2011; 3:e20.

3. Tang H, Sun H, Chen H, et al. Clinicopathological analysis of metaplastic meningioma: report of 15 cases in Huashan Hospital. Chin J Cancer Res. 2013; 25:112–118.
4. Matyja E, Naganska E, Zabek M, Jagielski J. Meningioma with the unique coexistence of secretory and lipomatous components: a case report with immunohistochemical and ultrastructural study. Clin Neuropathol. 2005; 24:257–261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download