Abstract
Extremely massive sellar xanthogranuloma (XG) are rare, and the surgical outcome and prognosis are not well known. XG remain unknown whether they are derived from Rathke's cleft cysts (RCCs) or craniopharyngiomas (CPs) following extensive inflammation and metaplasia, to the point that no epithelium is readily identifiable. These lesions usually tend to occur in younger patients (mean 28.3 years), have a smaller diameter, and remain primarily intrasellar region with infrequent calcification. This 36-year-old man presented our hospital with visual deterioration. At the time of visit, there were no neurological problems other than visual field defect and hormonal disorder. He visited our hospital in 2007 due to headache and decreased vision, and underwent transphenoid surgery for pituitary RCC. Since then, he has received treatment at our hospital for postoperative hormonal disorders. Through preoperative imaging study, the author suspected CP and underwent surgery. During the operation, the adhesion of the tumor to the surrounding major neurovascular structures was severe in the naked eyes, but the tumor could be removed more easily than expected. The postoperative histological findings were confirmed as XG. The postoperative course was uneventful. Compared to the previous literature, this case is a case where the size of XG is very large in a sellar region and it can be proved that it originated from the RCC. And regular follow-up is necessary to confirm the prognosis after surgery.
Xanthogranuloma (XG) accounts for 1.6–7% of incidental intracranial lesions discovered on autopsy and XG in the sellar and parasellar region is exceedingly rare [1]. The pathogenesis and exact cause of XG are unknown. Until recently, XG was not included in the WHO classification of central nervous system tumors. XGs are usually small in the sellar region and giant XGs with diameters of greater than 4 cm are extremely rare. The complex anatomy associated with giant XG and the surrounding neurovascular structures can make surgical excision difficult and may increase the risks associated with surgery. There are no accurate reports on the postoperative results, complications, or prognosis of giant XG.
In this paper, we report a case of giant XG that was completely resected without postoperative complications. In addition, we provide evidence supporting the hypothesis that XG originates from Rathke's cleft cyst (RCC). We performed a literature review of all cases of giant XG in the sellar region and described the associated clinical presentations, surgical outcomes, and follow-up results.
A 36-year-old man visited our hospital in 2007 due to decreased vision and underwent transphenoidal surgery for pituitary RCC. While receiving treatment at our hospital for hormonal disorders (panhypopituitarism), he presented 2 years after his initial surgery complaining of headache and visual deterioration. At the time of the visit, there were no neurological problems other than a visual field defect and hormonal disorder.
Sellar MRI without contrast revealed a huge, 3.0×4.6×4.6 cm, heterogeneous mass within the sellar region extending up to the third ventricle, right mesial temporal lobe, and retrosellar region (Fig. 1). The suprasellar mass was lobulated with predominantly heterogeneous solid-cystic components on T2-weighted and T1-weighted images. The mass demonstrated relative rim enhancement after gadolinium contrast. There was marked compression and superior displacement of the optic chiasm. There was no evidence of edema in the adjacent parenchyma. A head CT without contrast was obtained and showed a hypodense lesion with wall calcification and erosion of the dorsum sellae (Fig. 1). From the preoperative imaging studies, we suspected craniopharyngioma (CP) and performed surgery. We selected a right orbitozygomatic approach due to the severe optic nerve damage and prominent lateral and retrosellar extension of the tumor. During surgery, we observed severe adhesion of the tumor to the optic nerve, optic chiasm, and the cavernous segment of internal carotid artery with the naked eyes, but the tumor was removed more easily than expected. When the capsule was opened, yellowish-to-brown fluid was found. The pituitary stalk was also well preserved. The tumor is thought to have originated in the sella region.
The postoperative course was uneventful, and the patient's vision slowly improved although it never completely recovered. His preoperative hormonal deficiencies did not improve and continued to be controlled with medications. The postoperative histological findings confirmed to be XG (Fig. 2). Follow-up MRI was performed once a year, and the patient regularly visited our clinic for 6 years after surgery (Fig. 3). Recurrence was not observed during the follow-up period.
The most common intracranial site for XG is the choroid plexus, whereas XG is very rare in the sellar and suprasellar regions [2]. We defined giant XG as a lesions with a diameter greater than 4 cm. Including the present case, only 2 cases of giant sellar XG have been reported. Therefore, most preoperative lesions are presumed to be CPs or RCCs.
We consulted Medline PubMed databases to investigate articles related to sellar XG over the past 10 years. Including our patient, we analyzed the clinical features of 29 patients from the papers published in English from 2008 to 2017 [1234567]. The patients included 15 males and 14 females, with a mean age of 28.3 years (range, 5–60 years). Twenty of 29 patients (69%) presented with headache, 15 (52%) had endocrine disturbances, 12 (41%) had visual field disturbances, and one (3%) had diplopia. The lesions were resected via a transsphenoidal approach in 20 patients, and a transcranial approach was used in the other 9 patients. Recurrence occured in only one case over the follow-up periods ranging from 3 to 37 months (mean, 12 months). In most cases, the prognoses were favorable regardless of age, size and location of tumor, surgical approach, and resectability. While the postoperative visual function was improved in our patient, there was no significant change in his hormonal dysfunction.
The origin of sellar XG remains unclear. In 1999, Paulus et al. [8] identified 37 sellar XGs with different histopathologic features and clinical characteristics among 110 patients with adamantinomatous CP. The cholesterol crystals and foreign-body reactions in the cyst walls of XG were histologically similar to radicular cysts, whereas adamantinomatous CP closely resembled odontogenic tumors. XGs and CPs also differed in location, patient age, symptom, and prognosis. Therefore Paulus et al. [8] proposed that XGs represented a distinct entity that is different from both adamantinomatous and papillary CP.
It has also been hypothesized that sellar XG may be represent a secondary reaction caused by the inflammation, hemorrhage, and degeneration of RCC [19]. Ossification has also been associated with secondary granulomatous reactions to RCC [10]. Hama et al. [11] described 20 cases of RCC, 10 of which had inflammatory penetration of the cyst epithelium or subjacent stroma. RCCs were occasionally accompanied by foreign body inflammation around the cyst wall. As a result, sellar XG could be distinguished from adamantinomatous CP, and its characteristics were very similar to those of RCC with inflammatory changes [912]. These findings can be applied to our case, which derived from a secondary progression of RCC. Although the evidence presented above suggest a RCC origin for these lesions, it is not conclusive.
Preoperative differentiation between CP and RCC based on CT and MRI findings is difficult. The cholesterol clefts of a XG may appear hyper-intense in T1-weighted images, although the typical radiological signs of XG remain undefined [1314]. Sugata et al. [15] noted that severe hypopituitarism, hypointensity on T2-weighted MRI imaging, and absence of calcification may also support a preoperative diagnosis of XG. However, the calcification was observed in our case.
Histologically, XG lesions exhibit accumulation of foamy macrophages, multinucleated foreign body giant cells, cholesterol clefts, lymphocytic infiltration, hemosiderin deposits, and fibrous proliferation [8]. Positive immunohistochemical staining for β-catenin is also a reliable marker for differentiation between tumors and cysts of the sellar region. Nuclear β-catenin is found in most adamantinomatous CPs but is absent in papillary CPs and RCCs. Moreover, the ciliated cuboidal and columnar epithelium of RCC indicates that β-catenin immunoreactivity is restricted to the cell membrane [16]. A similar distribution is observed in the fine membranes of arachnoid cysts and cellular elements of XGs [17]. The present case supports the hypothesis that sellar XGs have distinct pathogenic etiologies from CPs, and the two entities should be clearly distinguished (Fig. 2).
In conclusion, our findings indicate that surgical treatment is feasible for any size XG lesion, and the prognosis could be good. In a case of recurrent RCC, XG might be included in differential diagnosis. There is no clear evidence regarding the origin of XG, and so further studies are needed.
Figures and Tables
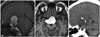 | Fig. 1Preoperative MRI and CT scans. A: T1-weighted imaging reveals heterogeneous intensity of the tumor. B: Sellar tumor extends to the right mesial temporal lobe and prepeduncular cistern on the T1-weighted image. C: Preoperative CT shows calcification in the superior and posterior cystic wall of the tumor. |
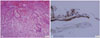 | Fig. 2Pathological findings. A: Foamy macrophages are found around cholesterol clefts. Inflammatory infiltrates are seen within dense fibrous tissues (hematoxylin and eosin, original magnification ×100). B: Epithelial membranes of the cyst wall are clearly positive for β-catenin (original magnification ×400). |
References
1. Rahmani R, Sukumaran M, Donaldson AM, Akselrod O, Lavi E, Schwartz TH. Parasellar xanthogranulomas. J Neurosurg. 2015; 122:812–817.

2. Liu ZH, Tzaan WC, Wu YY, Chen HC. Sellar xanthogranuloma manifesting as obstructive hydrocephalus. J Clin Neurosci. 2008; 15:929–933.

3. Kamoshima Y, Sawamura Y, Motegi H, Kubota K, Houkin K. Xanthogranuloma of the sellar region of children: series of five cases and literature review. Neurol Med Chir (Tokyo). 2011; 51:689–693.

4. Nishiuchi T, Murao K, Imachi H, et al. Xanthogranuloma of the intrasellar region presenting in pituitary dysfunction: a case report. J Med Case Rep. 2012; 6:119.

5. Hernández-Estrada RA, Kshettry VR, Vogel AN, Curtis MT, Evans JJ. Cholesterol granulomas presenting as sellar masses: a similar, but clinically distinct entity from craniopharyngioma and Rathke's cleft cyst. Pituitary. 2017; 20:325–332.

6. Yang B, Yang C, Fang J, et al. Clinicoradiologic features and surgical outcomes of sellar xanthogranulomas: a single-center 10-year experience. World Neurosurg. 2017; 99:439–447.

7. Dai CX, Guo XS, Liu XH, et al. Xanthogranuloma of the sellar region. Chin Med J (Engl). 2017; 130:249–250.
8. Paulus W, Honegger J, Keyvani K, Fahlbusch R. Xanthogranuloma of the sellar region: a clinicopathological entity different from adamantinomatous craniopharyngioma. Acta Neuropathol. 1999; 97:377–382.

9. Amano K, Kubo O, Komori T, et al. Clinicopathological features of sellar region xanthogranuloma: correlation with Rathke's cleft cyst. Brain Tumor Pathol. 2013; 30:233–241.

10. Miyajima Y, Oka H, Utsuki S, Fujii K. Rathke's cleft cyst with xanthogranulomatous change--case report. Neurol Med Chir (Tokyo). 2011; 51:740–742.
11. Hama S, Arita K, Nishisaka T, et al. Changes in the epithelium of Rathke cleft cyst associated with inflammation. J Neurosurg. 2002; 96:209–216.

12. Le BH, Towfighi J, Kapadia SB, Lopes MB. Comparative immunohistochemical assessment of craniopharyngioma and related lesions. Endocr Pathol. 2007; 18:23–30.

13. Arai A, Nishihara M, Sasayama T, et al. Xanthogranuloma of the sellar region--case report. Neurol Med Chir (Tokyo). 2010; 50:488–491.
14. Jung CS, Schänzer A, Hattingen E, Plate KH, Seifert V. Xanthogranuloma of the sellar region. Acta Neurochir (Wien). 2006; 148:473–477.

15. Sugata S, Hirano H, Yatsushiro K, Yunoue S, Nakamura K, Arita K. Xanthogranuloma in the suprasellar region. Neurol Med Chir (Tokyo). 2009; 49:124–127.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download