Abstract
Brain tumors represent a diverse spectrum of histology, biology, prognosis, and treatment options. Although MRI remains the gold standard for morphological tumor characterization, positron emission tomography (PET) can play a critical role in evaluating disease status. This article focuses on the use of PET with radiolabeled glucose and amino acid analogs to aid in the diagnosis of tumors and differentiate between recurrent tumors and radiation necrosis. The most widely used tracer is 18F-fluorodeoxyglucose (FDG). Although the intensity of FDG uptake is clearly associated with tumor grade, the exact role of FDG PET imaging remains debatable. Additionally, high uptake of FDG in normal grey matter limits its use in some low-grade tumors that may not be visualized. Because of their potential to overcome the limitation of FDG PET of brain tumors, 11C-methionine and 18F-3,4-dihydroxyphenylalanine (FDOPA) have been proposed. Low accumulation of amino acid tracers in normal brains allows the detection of low-grade gliomas and facilitates more precise tumor delineation. These amino acid tracers have higher sensitivity and specificity for detecting brain tumors and differentiating recurrent tumors from post-therapeutic changes. FDG and amino acid tracers may be complementary, and both may be required for assessment of an individual patient. Additional tracers for brain tumor imaging are currently under development. Combinations of different tracers might provide more in-depth information about tumor characteristics, and current limitations may thus be overcome in the near future. PET with various tracers including FDG, 11C-methionine, and FDOPA has improved the management of patients with brain tumors. To evaluate the exact value of PET, however, additional prospective large sample studies are needed.
Brain tumors can originate from different cells both from within the brain and from systemic tumors that have metastasized to the brain. Primary brain tumors most commonly arise from glial cells [1]. With an annual age-adjusted incidence rate of 28 per 100,000 in adults, gliomas account for approximately 27.2% of all brain and other central nervous system tumors, and approximately 81.3% of all malignant tumors [2].
Gliomas can be categorized into different pathologic subtypes. In addition to the pathologic type, World Health Organization classifications also provide histologic grades based on cellular alterations related to cancer aggressiveness. Grades I and II are considered low-grade tumors that have a prolonged clinical course. Grade III and IV tumors are considered high-grade lesions rapidly leading to death when left untreated [3].
Despite multimodal treatment strategies, the prognosis for patients with glioma is poor. The median survival for patients varies according to tumor grade, location, and age at diagnosis. Therefore, adequate tumor diagnosis and grading is thus crucial to initiate appropriate treatment and improve long-term outcomes [4].
MRI with gadolinium contrast enhancement is the gold standard imaging modality for assessing the morphological characteristics of brain tumors, such as location, mass effect, and contrast enhancement; however, it has several limitations. It cannot always distinguish gliomas from non-neoplastic lesions such as those resulting from vascular processes or inflammatory reactions. Because the absence of contrast enhancement does not always correspond to low-grade tumors, MRI is not perfect for grading gliomas. Furthermore, distinguishing tumor recurrence from post-surgical or post-radiotherapeutic changes remains a major challenge in brain imaging studies [5]. In recent decades, molecular imaging with positron emission tomography (PET) has gained increasing importance in identifying and delineating areas of increased tumor growth activity. Various PET tracers have been developed to visualize tumors using the hallmarks of cancers, such as metabolic derangement and replicative immortality. The tracer 18F-fluorodeoxyglucose (FDG) visualizes glucose metabolism, radiolabeled amino acids [e.g., 11C-methionine, 18F-3,4-dihydroxyphenylalanine (FDOPA), and O-(2-18F-Fluoroethyl)-l-Tyrosine (FET)] perform protein synthesis, and 18F-fluorothymidine (FLT) performs DNA replications. PET fused with computed tomography (PET/CT) can obtain detailed anatomical information on PET results and provides clinically invaluable information regarding primary detection and differentiation between various underlying tumor types, initial tumor grading and risk stratification, therapy planning, selection of biopsy site, response evaluation, and recurrence detection [678]. The current article discusses some of the positive aspects of the contemporary use of PET or PET/CT in primary brain tumors.
FDG PET imaging was first used to detect and differentiate between low- and high-grade tumors [9]. Similar to most malignancies elsewhere in the body, malignant brain tumors generally have increased glucose metabolism and increased FDG uptake, and FDG is actively transported across the intact blood-brain barrier (BBB) (Fig. 1). Anaerobic glycolysis has been shown to occur in advanced cancers, even with an abundance of oxygen, a process named the Warburg effect. The high glycolytic rate of cancerous lesions results from various biological changes, including high levels of the membrane glucose transporter and increased cytosolic glycolytic enzymes such as hexokinase. Consequently, the greater demand for glycolytic substrates causes increased transport of the glucose analog FDG into malignant cells [101112].
FDG PET can be used to identify differences in glucose uptake among healthy brains, low- and high-grade gliomas, and radionecrosis [1314]. FDG uptake is generally considered to reflect both tumor cell viability and density, and is directly related to tumor grade [1516]. FDG uptake in low-grade tumors is similar to that of white matter, whereas Grade III and IV tumors exhibit glucose metabolic activity comparable to or higher than that of grey matter (Fig. 2). A meta-analysis conducted by Zhao et al. [17] revealed that FDG PET was able to detect brain tumors with a sensitivity of 71% and a specificity of 77%, whereas another study on detecting high-grade gliomas found that FDG PET had a sensitivity of 94% and a specificity of 77% [9]. Because the similarities in glucose metabolic activity between tumors and grey matter cause difficulties in the analysis of FDG-PET images, several studies have shown that delaying scanning times by 3 hours after FDG injection considerably improves the contrast between malignant brain tumors and normal brain tissue [1819].
Because treatment-induced changes such as radionecrosis suband post-surgical changes are highly difficult to distinguish from tumor recurrence, evaluation of disease status after treatment is challenging with MRI alone [20]. Conversely, FDG PET can detect recurrent high-grade tumors. Chao et al. [14] reported sensitivity of 75% and specificity of 81% for FDG PET in differentiating recurrent tumors from post-radiation changes. They also observed an improvement in the sensitivity of tumor recurrence detection after stereotactic radiotherapy, from 65% to 85%, when FDG PET was added to standard MRI. Previous studies have reported high sensitivities and specificities for FDG PET of 81–86% and 40–94%, respectively, for distinguishing radionecrosis from residual or recurrent tumors whereas those for contrast enhanced MRI were 95% and 23%, respectively [2122].
Wang et al. [23] defined the criteria for positive and negative FDG PET scans as tracer uptake above or below the expected uptake in the adjacent brain tissue, which achieved high overall sensitivity and accuracy of 80% and 87%, respectively, with regard to differentiating recurrent tumors from post-radiation changes.
However, values of FDG PET are inherently limited by the FDG avidity of normal brain tissue. The physiologic glucose consumption in the normal brain generates a high background uptake of FDG, which is generally high in gray matter, and moderate to high in white matter [242526]. In addition, various non-malignant intracerebral lesions also have varying levels of increased FDG uptake (e.g., with inflammatory or infectious causes), and this also applies to normal brain tissue adjacent to tumor lesions. Thus, differentiating between malignant and non-malignant causes of increased FDG uptake is difficult [272829].
Although FDG remains the most widely used radiotracer for PET imaging, radiopharmaceutical development is an evolving domain, promising higher sensitivity as well as higher specificity for certain tumor entities [30]. Because of physiologically low uptake in healthy brain tissue and absent or low uptake in inflammatory lesions, radiolabeled amino acids or their analogs have been demonstrated to overcome the limitations of FDG [3132] .
Because of the limitations of FDG PET in assessing brain tumors, amino acid-based radiotracers have been developed. The most popular amino acid tracer is 11C-methionine, which has been investigated in many studies on brain tumors (Fig. 3). The use of 11C-methionine provides a high detection rate for brain tumors and good lesion delineation because of the low physiological uptake of the amino acid in healthy brains with high contrast between normal and cancerous tissue [333435363738]. Increased 11C-methionine uptake is associated with upregulation of L-type amino acid transporter 1 (LAT1) and proliferation of the tumor microvasculature [39404142]. Although methionine PET has been shown to have high sensitivity for gliomas, false-positive results may be seen under benign conditions, such as cases of demyelination, leukoencephalitis, or abscess [43].
Several studies diagnosing untreated brain tumors with methionine PET have reported relatively high sensitivities, ranging from 76% to 91%, and specificities ranging from 75% to 100% [353844454647]. A recent meta-analysis found a 91% sensitivity and an 86% specificity [17]. Methionine PET is more suitable than FDG PET alone for diagnosing and managing patients, particularly those with low-grade tumors [384849].
In high-grade gliomas, tracer leakage from a disrupted BBB contributes considerably to amino acid uptake. However, in low-grade gliomas, amino acid uptake occurs without substantial BBB breakdown, corresponding to an upregulation of LAT1 [45]. Therefore, the relationship between tumor grade and the intensity of amino acid analog uptake remains subject to speculation; some studies have reported strong correlation between the two parameters [505152], whereas others have reached the opposite conclusion [535455].
Methionine PET can also detect recurrent tumors with high sensitivity and specificity, allowing differentiation between tumor recurrence and radionecrosis. A recent meta-analysis of methionine PET reported a summary sensitivity of 70% and specificity of 93% for high-grade gliomas in the detection of recurrent tumors [56].
However, because of the short half-life of 11C, 18F-labeled amino acid tracers were developed, such as FDOPA and FET [4315758]. Whereas FDOPA is widely spread in the United States, FET is more common in Europe [59].
Similar to radiolabeled methionine, uptake of FDOPA is mediated by amino acid transporters and does not require disruption of the BBB. Therefore, FDOPA and 11C–methionine have similar distribution in tumors [6061].
Despite published series having involved mixed patients populations, FDOPA PET reportedly has high sensitivity and specificity for detecting brain tumors, ranging from 85% to 100% and from 86% to 90%, respectively [60626364]. Accumulation of FDOPA does not vary substantially within different tumor grades, and the amino acid analog is clearly superior to 18F-FDG for diagnosing low- and high-grade gliomas [6465].
Because FDOPA uptake in brain tumors does not depend on the BBB, delineation of tumor extent is reportedly more accurate, and areas with increased uptake on PET are often larger than areas with contrast-enhanced lesions on MRI [66]. Therefore, amino acid PET can be useful for treatment planning, and Grosu et al. [67] reported better outcomes for patients with radiotherapy planned on the basis of tumor extent as defined using amino acid PET.
FDOPA PET provides crucial information for the detection of recurrent brain tumors as well as initial diagnosis. It is a valuable tool for treatment monitoring because it helps in assessing treatment response and evaluating patient prognosis after therapy. Previous studies have reported sensitivity and specificity of FDOPA PET for detecting tumor recurrence as ranging from 90% to 92% and from 92% to 95%, respectively [326869].
The pyrimidine analog 3′-deoxy-3′-FLT has been studied as a marker of tumor proliferation rate by reflecting thymidine kinase-1 activity, which is the principle enzyme in the pathway of DNA synthesis. Because no transporter has sufficient capacity, uptake of FLT in the brain depends on BBB permeability. In brain tumors with a damaged BBB, therefore, FLT provides highly reliable tumor-to-background contrast but cannot be used in low-grade gliomas with an intact BBB [7071].
Whereas the sensitivity of FLT PET for detecting high-grade gliomas can reach 100%, a lower overall sensitivity of 83% has been shown because of major differences in uptake between high- and low-grade tumors [7273]. Hence, the sensitivity of all grades is typically lower than with FDG PET [74] and methionine PET [75]. Conversely, FLT PET seems to be superior to methionine PET in tumor grading and assessment of proliferation activity in gliomas of different grades [7677].
Because the information gained by different imaging methods is complementary and brain PET scans generally should not be interpreted without access to the corresponding MRI scans, combining all imaging methods might provide optimal results for assessment of tumor characteristics [787980818283]. Combined PET/MR can readily be achieved using standard software and is provided more directly and conveniently by hybrid PET/MR machines (Fig. 1, 2, 3). Although the effect of image fusion does not play an essential role in the case of brain imaging, accurate image fusion can be easily obtained through image co-registration based on fixed points. PET/MR also has the advantage of low radiation exposure compared to PET/CT, rendering it particularly attractive for pediatric patients.
The nitroimidazole derivative tracer 18F-Fluoromisonidazole (F-MISO) has been developed as a PET tracer, to visualize intratumoral hypoxic areas before and during radiation therapy [8485]. In addition, F-MISO is able to diffuse freely across the BBB, it is useful imaging tracer for brain tumor. Dual-phase F-MISO PET has been used; early F-MISO distribution reflects blood flow, while later tracer is accumulated in hypoxic area [8687]. Hypoxia measurements have been shown to correlate with invasion, tumor recurrence, the probability of metastatic spread and decreased patient survival as well as resistance to radiation and chemotherapy. However, the biggest obstacle for using F-MISO is limited availability, and further clinical studies are still needed for verifying clinical usefulness of F-MISO PET. Nevertheless, the majority of PET studies have been limited to small sample size and retrospective designs, lacking comparability because of different acquisition and data evaluation methods. Therefore, the clinical value of PET in brain tumors might still be underestimated. Multicenter clinical trials of PET are crucial to elucidate the optimal PET setting for assessing brain tumors, which can be useful for guiding optimal diagnostic and therapeutic decision making and ultimately improving the prognosis of brain tumors.
Additional tracers for brain tumor imaging are under active development, and PET tracers using other metabolic processes, such as phospholipid membrane biosynthesis, hypoxia, receptor binding, and oxygen metabolism and blood flow, will be crucial for forming personalized therapeutic strategies using targeted agents. The combination of different tracers might provide more accurate information on the characteristics of various brain tumors, and the current limitations may thus be overcome in the near future.
PET imaging with oncologic radiotracers can visualize various biological statuses of brain tumors and improves diagnostic and therapeutic planning in certain patients with brain tumors. Advancement of PET chemistry and development of imaging technologies will broaden the applications of PET imaging in the field of brain tumors.
Figures and Tables
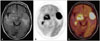 | Fig. 1FDG PET/MR for CNS lymphoma. 79-year-old woman diagnosed as CNS lymphoma. T2 fluid attenuated inversion recovery MRI shows multiple lesions with high signal in both hemisphere (A). FDG PET (B) and FDG PET/MR (C) show intense tracer uptake at the lesions. FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography; CNS, central nervous system. |
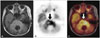 | Fig. 2FDG PET/MR for high-grade glioma. 18-year-old woman diagnosed as a glioblastoma, WHO grade IV. T2 fluid attenuated inversion recovery MRI shows high signal in pontine lesion (A). FDG PET (B) and FDG PET/MR (C) show increased tracer uptake at the lesion (arrows). FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography. |
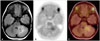 | Fig. 311C-methionine PET/MR. 5-year-old girl diagnosed low-grade glioma in cerebellum. T2 fluid attenuated inversion recovery MRI shows high signal in a cerebellar lesion (A). 11C-methionine PET (B) and 11C-methionine PET/MR (C) show increased tracer uptake at the cerebellar lesion. PET, positron emission tomography. |
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the South Korea government (2014R1A5A2009242).
The authors would like to thank professor Jin Chul Paeng (Seoul National University Hospital, Seoul, Korea) for providing PET/MR images.
References
1. Segtnan EA, Hess S, Grupe P, Høilund-Carlsen PF. 18F-fluorodeoxyglucose PET/computed tomography for primary brain tumors. PET Clin. 2015; 10:59–73.

2. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017; 19:suppl_5. v1–v88.

3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803–820.

4. Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, Prior JO. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. 2016; 18:426–434.

6. El-Deiry WS, Sigman CC, Kelloff GJ. Imaging and oncologic drug development. J Clin Oncol. 2006; 24:3261–3273.

7. la Fougère C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011; 13:806–819.

8. Segtnan EA, Grupe P, Jarden JO, et al. Prognostic implications of total hemispheric glucose metabolism ratio in cerebrocerebellar diaschisis. J Nucl Med. 2017; 58:768–773.

9. Delbeke D, Meyerowitz C, Lapidus RL, et al. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology. 1995; 195:47–52.

11. Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978; 19:1154–1161.

12. Nishioka T, Oda Y, Seino Y, et al. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992; 52:3972–3979.

13. Singhal T, Narayanan TK, Jacobs MP, Bal C, Mantil JC. 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Nucl Med. 2012; 53:1709–1715.

14. Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001; 96:191–197.

15. Padma MV, Said S, Jacobs M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003; 64:227–237.

16. Herholz K, Pietrzyk U, Voges J, et al. Correlation of glucose consumption and tumor cell density in astrocytomas. A stereotactic PET study. J Neurosurg. 1993; 79:853–858.

17. Zhao C, Zhang Y, Wang J. A meta-analysis on the diagnostic performance of (18)F-FDG and (11)C-methionine PET for differentiating brain tumors. AJNR Am J Neuroradiol. 2014; 35:1058–1065.

18. Horky LL, Hsiao EM, Weiss SE, Drappatz J, Gerbaudo VH. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J Neurooncol. 2011; 103:137–146.
19. Prieto E, Martí-Climent JM, Domínguez-Prado I, et al. Voxel-based analysis of dual-time-point 18F-FDG PET images for brain tumor identification and delineation. J Nucl Med. 2011; 52:865–872.
20. Wong TZ, van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am. 2002; 12:615–626.
21. Santra A, Kumar R, Sharma P, et al. F-18 FDG PET-CT in patients with recurrent glioma: comparison with contrast enhanced MRI. Eur J Radiol. 2012; 81:508–513.
22. Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med. 2000; 41:1861–1867.

23. Wang SX, Boethius J, Ericson K. FDG-PET on irradiated brain tumor: ten years' summary. Acta Radiologica. 2006; 47:85–90.

24. Sharma A, McConathy J. Overview of PET tracers for brain tumor imaging. PET Clin. 2013; 8:129–146.

26. Chen W, Silverman DH. Advances in evaluation of primary brain tumors. Semin Nucl Med. 2008; 38:240–250.
27. Salber D, Stoffels G, Pauleit D, et al. Differential uptake of O-(2-18F-fluoroethyl)-L-tyrosine, L-3H-methionine, and 3H-deoxyglucose in brain abscesses. J Nucl Med. 2007; 48:2056–2062.
28. Morbelli S, Djekidel M, Hesse S, Pagani M, Barthel H. Role of (18)F-FDG-PET imaging in the diagnosis of autoimmune encephalitis. Lancet Neurol. 2016; 15:1009–1010.
29. Solnes LB, Jones KM, Rowe SP, et al. Diagnostic value of 18F-FDG PET/CT versus MRI in the setting of antibody-specific autoimmune encephalitis. J Nucl Med. 2017; 58:1307–1313.
30. Koopmans KP, Glaudemans AW. Rationale for the use of radiolabelled peptides in diagnosis and therapy. Eur J Nucl Med Mol Imaging. 2012; 39:Suppl 1. S4–S10.

31. Gulyás B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging. 2012; 56:173–190.

32. Karunanithi S, Sharma P, Kumar A, et al. Can (18)F-FDOPA PET/CT predict survival in patients with suspected recurrent glioma? A prospective study. Eur J Radiol. 2014; 83:219–225.

33. Mosskin M, Ericson K, Hindmarsh T, et al. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol. 1989; 30:225–232.

34. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008; 49:694–699.

35. Ullrich RT, Kracht L, Brunn A, et al. Methyl-L-11C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J Nucl Med. 2009; 50:1962–1968.

36. Kobayashi K, Hirata K, Yamaguchi S, et al. Prognostic value of volume-based measurements on (11)C-methionine PET in glioma patients. Eur J Nucl Med Mol Imaging. 2015; 42:1071–1080.

37. Glaudemans AW, Enting RH, Heesters MA, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013; 40:615–635.

38. Kracht LW, Miletic H, Busch S, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004; 10:7163–7170.

39. Ishiwata K, Kubota K, Murakami M, et al. Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J Nucl Med. 1993; 34:1936–1943.

40. Kato T, Shinoda J, Oka N, et al. Analysis of 11C-methionine uptake in low-grade gliomas and correlation with proliferative activity. AJNR Am J Neuroradiol. 2008; 29:1867–1871.
41. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010; 9:906–920.
42. Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol. 2008; 10:1–18.
43. Singhal T, Alavi A, Kim CK. Brain: positron emission tomography tracers beyond [18F]fluorodeoxyglucose. PET Clin. 2014; 9:267–276.
44. Braun V, Dempf S, Weller R, Reske SN, Schachenmayr W, Richter HP. Cranial neuronavigation with direct integration of (11)C methionine positron emission tomography (PET) data- results of a pilot study in 32 surgical cases. Acta Neurochir (Wien). 2002; 144:777–782. discussion 782.

45. Herholz K, Hölzer T, Bauer B, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998; 50:1316–1322.

46. Yamane T, Sakamoto S, Senda M. Clinical impact of (11)C-methionine PET on expected management of patients with brain neoplasm. Eur J Nucl Med Mol Imaging. 2010; 37:685–690.

47. Dandois V, Rommel D, Renard L, Jamart J, Cosnard G. Substitution of 11C-methionine PET by perfusion MRI during the follow-up of treated high-grade gliomas: preliminary results in clinical practice. J Neuroradiol. 2010; 37:89–97.

48. Mosskin M, von Holst H, Bergström M, et al. Positron emission tomography with 11C-methionine and computed tomography of intracranial tumours compared with histopathologic examination of multiple biopsies. Acta Radiol. 1987; 28:673–681.

49. Demetriades AK, Almeida AC, Bhangoo RS, Barrington SF. Applications of positron emission tomography in neuro-oncology: a clinical approach. Surgeon. 2014; 12:148–157.

50. De Witte O, Goldberg I, Wikler D, et al. Positron emission tomography with injection of methionine as a prognostic factor in glioma. J Neurosurg. 2001; 95:746–750.

51. Kameyama M, Shirane R, Itoh J, et al. The accumulation of 11C-methionine in cerebral glioma patients studied with PET. Acta Neurochir (Wien). 1990; 104:8–12.

52. Kato T, Shinoda J, Nakayama N, et al. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. AJNR Am J Neuroradiol. 2008; 29:1176–1182.

53. Kaschten B, Stevenaert A, Sadzot B, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med. 1998; 39:778–785.

54. Ceyssens S, Van Laere K, de Groot T, Goffin J, Bormans G, Mortelmans L. [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. AJNR Am J Neuroradiol. 2006; 27:1432–1437.
55. Moulin-Romsée G, D'Hondt E, de Groot T, et al. Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur J Nucl Med Mol Imaging. 2007; 34:2082–2087.
56. Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol. 2013; 34:944–950. S1–S11.

57. Bergmann R, Pietzsch J, Fuechtner F, et al. 3-O-methyl-6-18F-fluoro-L-dopa, a new tumor imaging agent: investigation of transport mechanism in vitro. J Nucl Med. 2004; 45:2116–2122.

58. Heiss WD, Wienhard K, Wagner R, et al. F-Dopa as an amino acid tracer to detect brain tumors. J Nucl Med. 1996; 37:1180–1182.

59. Suchorska B, Tonn JC, Jansen NL. PET imaging for brain tumor diagnostics. Curr Opin Neurol. 2014; 27:683–688.

60. Becherer A, Karanikas G, Szabó M, et al. Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003; 30:1561–1567.

61. Bell C, Dowson N, Puttick S, et al. Increasing feasibility and utility of (18)F-FDOPA PET for the management of glioma. Nucl Med Biol. 2015; 42:788–795.

62. Fueger BJ, Czernin J, Cloughesy T, et al. Correlation of 6-18F-fluoro-L-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. J Nucl Med. 2010; 51:1532–1538.

63. Karunanithi S, Sharma P, Kumar A, et al. 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: prospective comparison with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013; 40:1025–1035.

64. Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006; 47:904–911.

65. Beuthien-Baumann B, Bredow J, Burchert W, et al. 3-O-methyl-6-[18F]fluoro-L-DOPA and its evaluation in brain tumour imaging. Eur J Nucl Med Mol Imaging. 2003; 30:1004–1008.

66. Pafundi DH, Laack NN, Youland RS, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013; 15:1058–1067.
67. Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005; 63:511–519.
68. Schwarzenberg J, Czernin J, Cloughesy TF, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014; 20:3550–3559.

69. Cicone F, Minniti G, Romano A, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging. 2015; 42:103–111.

70. Herholz K. Brain tumors: an update on clinical PET research in gliomas. Semin Nucl Med. 2017; 47:5–17.

71. Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005; 46:945–952.

72. Yamamoto Y, Ono Y, Aga F, Kawai N, Kudomi N, Nishiyama Y. Correlation of 18F-FLT uptake with tumor grade and Ki-67 immunohistochemistry in patients with newly diagnosed and recurrent gliomas. J Nucl Med. 2012; 53:1911–1915.

73. Nikaki A, Angelidis G, Efthimiadou R, et al. 18F-fluorothymidine PET imaging in gliomas: an update. Ann Nucl Med. 2017; 31:495–505.

74. Choi SJ, Kim JS, Kim JH, et al. [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging. 2005; 32:653–659.

75. Jeong SY, Lim SM. Comparison of 3′-deoxy-3′-[18F]fluorothymidine PET and O-(2-[18F]fluoroethyl)-L-tyrosine PET in patients with newly diagnosed glioma. Nucl Med Biol. 2012; 39:977–981.

76. Hatakeyama T, Kawai N, Nishiyama Y, et al. 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging. 2008; 35:2009–2017.

77. Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005; 46:1948–1958.

78. Filss CP, Galldiks N, Stoffels G, et al. Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med. 2014; 55:540–545.

79. Rahm V, Boxheimer L, Bruehlmeier M, et al. Focal changes in diffusivity on apparent diffusion coefficient MR imaging and amino acid uptake on PET do not colocalize in nonenhancing low-grade gliomas. J Nucl Med. 2014; 55:546–550.

80. Yoon JH, Kim JH, Kang WJ, et al. Grading of cerebral glioma with multiparametric MR imaging and 18F-FDG-PET: concordance and accuracy. Eur Radiol. 2014; 24:380–389.

81. Rausch I, Rischka L, Ladefoged CN, et al. PET/MRI for oncologic brain imaging: a comparison of standard MR-based attenuation corrections with a model-based approach for the Siemens mMR PET/MR System. J Nucl Med. 2017; 58:1519–1525.

82. Verger A, Filss CP, Lohmann P, et al. Comparison of 18F-FET PET and perfusion-weighted MRI for glioma grading: a hybrid PET/MR study. Eur J Nucl Med Mol Imaging. 2017; 44:2257–2265.
83. Neuner I, Kaffanke JB, Langen KJ, et al. Multimodal imaging utilising integrated MR-PET for human brain tumour assessment. Eur Radiol. 2012; 22:2568–2580.
84. Tachibana I, Nishimura Y, Shibata T, et al. A prospective clinical trial of tumor hypoxia imaging with 18F-fluoromisonidazole positron emission tomography and computed tomography (F-MISO PET/CT) before and during radiation therapy. J Radiat Res. 2013; 54:1078–1084.
85. Sachpekidis C, Thieke C, Askoxylakis V, et al. Combined use of (18)FFDG and (18)F-FMISO in unresectable non-small cell lung cancer patients planned for radiotherapy: a dynamic PET/CT study. Am J Nucl Med Mol Imaging. 2015; 5:127–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download