Abstract
Pheochromocytoma (PCC) is a neuroendocrine tumor that mainly arises from the medulla of the adrenal gland. Some PCCs become malignant and metastasize to other organs. For example, it typically involves skeletal system, liver, lung, and regional lymph nodes. However, only a few cases of PCC with brain metastasis have been reported worldwide. We report a case of metastatic brain tumor from PCC in South Korea in 2016. A 52-year-old man presented with headache, dizziness and motor aphasia. He had a medical history of PCC with multi-organ metastasis, previously underwent several operations, and was treated with chemotherapy and radiotherapy. Brain MRIs showed a brain tumor on the left parietal lobe. Postoperative pathology confirmed that the metastatic brain tumor derived from malignant PCC. This is the first report PCC with brain metastasis in South Korea.
Pheochromocytomas (PCCs) are uncommon, catecholamine-producing, neuroendocrine tumors that mainly arise from chromaffin cells in adrenal gland. Usually, PCCs are benign tumors, but some have clinically malignant characteristics. The prevalence of malignancy is commonly cited at about 10%, but other estimates suggest rates of between 5–26%, depending on how malignancy is defined [1]. Malignant PCCs are able to metastasize to other organs in absence of chromaffin cells. Malignant PCC often metastasize to the skeletal system, liver, lung, and regional lymph nodes [2]. Cerebral metastases in PCC are exceptionally rare. In this report, we present the unique case of brain metastasis from adrenal PCC, which has been managed by surgical resection and radiotherapy.
A 52-year-old man admitted on January 2017 with complaints of headache, dizziness and motor aphasia. On October 2015, he complained of left hip pain, and was diagnosed with a left adrenal cortical adenoma on abdomino-pelvic CT when examined at a local medical center. However, he had no surgical treatment, and follow-up CT showed a huge, left adrenal mass compressing spleen, pancreas and thrombus in inferior vena cava (IVC), right common iliac vein. The patient underwent left adrenalectomy, left nephrectomy, IVC reconstruction, thrombectomy, and distal subtotal pancreatectomy with splenectomy on January 2016. Thereafter, he was treated with metaiodobenzylguanidine therapy and underwent excision of the retroperitoneal cancer on June 2016. Despite 2 times of operations and radiotherapy sections, follow-up CT showed progressive disease, and he was treated with additional 3 cycles of combination chemotherapy with cyclophosphamide, vincristine and dacarbazine (CVD) and 2 cycles of combination chemotherapy with etoposide, ifosfamide and cisplatin (VIP-STS), but the tumor size did not reduce. In addition, the patient received mediastinal and subdiaphragm mass excision and diaphragm repair. The patient was scheduled to undergo CT follow-up and positron emission tomography-CT to evaluate cancer progression. However, the patient had symptoms of headache, dizziness and motor aphasia, and brain MRI showed multiple (more than 10) enhancing masses in the bilateral cerebral hemisphere and right pons. The largest one measured 5.7 cm, and featured a cystic mass with hemorrhagic components and perilesional edema in left parietal lobe, compressing left lateral ventricle inferiorly, and causing midline shift to the right. Some lesions showed hemorrhagic change (Fig. 1). We performed a left parietal craniotomy with gross total tumor removal by transcortical approach on February 2, 2017. While the patient was anesthetized, we performed a left parieto-occipital craniotomy and corticectomy by using a navigation device. Once the tumor margin was exposed and dissected with a CUSA and microdissector, the tumor was removed. On gross examination, a 5.8 cm sized hypervascular tumor was observed inside of the ventricle trigone when it was opened. PCC was diagnosed by frozen biopsy samples, and later confirming the diagnosis by examining tissue pathology. Postoperative MRI showed total removal of the largest mass in parietal area and a slightly improved midline shift. The size of other multiple metastatic lesions in the bilateral cerebral hemisphere, with or without hemorrhage, and the size of the right pons did not change (Fig. 2). However, the patient demonstrated right hemianopsia during the postoperative visual field test (Humphrey test). On day 7, the patient experienced severe headaches and visual field was blurred and we took emergent brain CT and revealed intracerebral hemorrhage (ICH) on right occipital area. Navigation guided ICH catheter insertion was performed immediately in the operating room. On day 10, ICH catheter was removed and discharged. Postoperative whole brain radiotherapy was scheduled in order to remove any residual metastatic lesions. Total of 3,000 cGy (300 cGy/fx×10 fx) radiotherapy was performed. Thereafter, follow-up brain MRI showed the partial decrease in size of multiple metastatic lesions, and no other newly appearing lesion. Unfortunately, the patient's intraabdominal cancer progressed further, and he expired. This case report was performed with informed consent according to protocols approved by Institutional Review Boards of Severance Hospital (4-2018-0790).
PCCs are usually benign tumors arising from chromaffin cells found mainly of the adrenal gland that may sometimes become malignant [3]. To establish malignancy in PCC requires evidence of metastases to nonchromaffin sites (not in adrenal medulla or sympathetic nervous ganglia). These sites include the skeletal system, liver, lung, and regional lymph nodes [4]. The 5-year survival rate in metastatic PCC is estimated at <50% [3]. Cerebral metastases in PCC are exceptionally rare [5678]. According to literature, a total of 3 cases have been reported so far. The first reported case was of a 49-year-old man in 1992 in France. Thereafter, a 31-year-old woman and a 53-year-old man were reported in 2001 and 2010, respectively [689]. Prior to this, there has been no case report of PCC with brain metastasis from Korea. The literature suggests that genetic alteration may be associated with cerebral metastasis in malignant PCCs. In certain patient groups with a mutation in the succinate dehydrogenase (SDH) B gene, malignancy is reported to occur in as many as 83% of patients [9]. In the malignant PCC with brain metastasis case reported in 2010, deletion of chromosome 18q is a unique characteristic compared to other malignant PCC cases. Histopathological morphology of PCC is a Zellballen growth pattern, separated by delicate, fibrovascular stroma. However, in some cases of PCC, the Zellballen pattern is not seen, and a diffuse growth type pattern is detected. In our cases, hematoxylin and eosin stain displayed atypical cells with eosinophilic cytoplasm and hyperchromatic nuclei surrounded by sustentacular cells and diffuse pattern (Fig. 3A, B). Staining for synaptophysin (neuroendocrine marker) is markedly positive for tumor cells and the surrounding sustentacular cells are positive for S-100 (Fig. 3C, D). Additionally, for differential diagnosis with a primary glial tumor, Oligo-2 immunohistochemical analysis was performed, and the tumor was found to be negative for Oligo-2 (Fig. 3E). These histopathological findings supported the diagnosis of PCC. Usually, PCCs release catecholamines causing hypertension, palpitation and sweating. As a result, blood or 24-h urine has elevated levels of catecholamines (metanephrine, dopamine, and vanillylmandelic acid). However, our patient had no typical cardiac symptoms and maintained catecholamine levels within the normal range during the course of the case study. In some rare cases, PCCs do not synthesize or release any catecholamines (non-functional tumors). Tumors due to SDH B gene mutations are often subclinical, poorly differentiated, contain low amounts of catecholamines, and are usually malignant at diagnosis [10]. Postoperative whole brain radiotherapy is used to treat PCC with brain metastasis. However, because of the rarity of these tumors, the prognosis of the disease and efficacy of radiotherapy is not clear.
Brain metastases from PCC are rarely reported. To the best of our knowledge, this is the first case in the literature, where a brain metastasis from PCC has been reported in Korea. And the possibility of metastasis to brain has not been excluded. Therefore, patients with malignant PCC and have neurological symptoms are needed to support the findings of the brain imaging study.
Figures and Tables
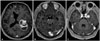 | Fig. 1Postcontrast axial T1-weighted magnetic resonance finding of intracranial metastatic PCC at preoperation. A: Largest one measuring 5.7 cm solid and cystic mass with hemorrhagic components and perilesional edema in left parietal lobe, compressing left lateral ventricle inferiorly. B and C: Multiple enhancing masses in bilateral cerebral hemisphere and right pons. |
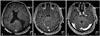 | Fig. 2Postcontrast axial T1-weighted magnetic resonance finding of intracranial metastatic PCC at postoperation. A: Postoperative acute ischemic change and hemorrhage along the resection margin left parietal, temporal and frontal lobe. Slightly improved midline shift. B and C: No change in size of other multiple metastatic lesions with or without hemorrhage in the bilateral cerebral hemisphere and right pons. |
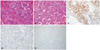 | Fig. 3Tumor histopathology. A and B: Atypical cells with eosinophilic cytoplasm and hyperchromatic nuclei (hematoxylin and eosin staining, original magnification ×400 and ×200). C: Positive for synaptophysin (immunostain, neuroendocrine marker, original magnification ×100). D: Positive for S-100 (immunostain, suscentacular cell marker, original magnification ×200). E: Negative for Oligo-2 (Immunostain, glial cell tumor marker, original magnification ×100). |
Acknowledgments
This study was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI17C2586) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP: Ministry of Science, ICT and Future Planning) (NRF-2017M2A2A7A01071036).
References
1. Mallory GW, Fang S, Giannini C, Van Gompel JJ, Parney IF. Brain carcinoid metastases: outcomes and prognostic factors. J Neurosurg. 2013; 118:889–895.

3. Eisenhofer G, Bornstein SR, Brouwers FM, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004; 11:423–436.

4. Thouënnon E, Elkahloun AG, Guillemot J, et al. Identification of potential gene markers and insights into the pathophysiology of pheochromocytoma malignancy. J Clin Endocrinol Metab. 2007; 92:4865–4872.

5. Ferrari G, Togni R, Pizzedaz C, Aldovini D. Cerebral metastases in pheochromocytoma. Pathologica. 1979; 71:703–710.
6. Gentile S, Rainero I, Savi L, Rivoiro C, Pinessi L. Brain metastasis from pheochromocytoma in a patient with multiple endocrine neoplasia type 2A. Panminerva Med. 2001; 43:305–306.
7. Melicow MM. One hundred cases of pheochromocytoma (107 tumors) at the Columbia-Presbyterian Medical Center, 1926–1976: a clinicopathological analysis. Cancer. 1977; 40:1987–2004.

8. Mornex R, Badet C, Peyrin L. Malignant pheochromocytoma: a series of 14 cases observed between 1966 and 1990. J Endocrinol Invest. 1992; 15:643–649.

9. Gimenez-Roqueplo AP, Favier J, Rustin P, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003; 63:5615–5621.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download