Dear Editor:
Recently, various stem cell lines have been widely used in tissue engineering. Among them, the mesenchymal stem cell (MSC) has been reported to stimulate wound healing. In particular, the bone marrow mesenchymal stem cell (BMSC) migrates directly towards a wound, differentiates into various cells and provides a favorable environment where other cells can regenerate quickly
1. The adipose-derived stem cell (ADSC) is another line that has features of the MSC. Although these two types of MSCs have great potential for treating wounds, few studies have investigated how they function in skin regeneration.
Activin A, a homodimer of two inhibin βA subunits linked by a disulfide bond, is a cytokine produced by various cell types including stem cells
2. It participates in regulation of stem cell maintenance
3, and also plays an important role in the wound healing process
4. Because the mesenchymal-epithelial interaction is a critical process for tissue regeneration, we focused on activin A as an important factor of dermal-epidermal interaction.
Recent studies have revealed that the epithelialization process of living skin equivalent (LSE) was similar to that occurring during the re-epithelialization process after wounding
5. Therefore, in our study, we compared three different LSE models that were cultured with ADSC, BMSC, and fibroblast. This study was approved by Seoul National University Bundang Hospital Institutional Review Board (IRB no. 1305/202-004 and 1207/164-003).
Three different LSE models (fibroblast-based LSE, BMSC-based LSE, and ADSC-based LSE) were reconstructed according to the methods described previously
6. For reconstruction of the LSE models; ADSC, BMSC, and fibroblast were added as dermal cells to prepare the dermal substitutes. After gelling of the dermal substitutes, the keratinocytes were seeded. Human keratinocytes and dermal fibroblasts then were isolated from foreskins obtained during child circumcision, and human BMSCs (PT-2501) and ADSCs (PT-5006) were purchased from Lonza, Inc., Walkersville, MD, USA.
To analyze the LSE, sections were stained with hematoxylin and eosin. The level of activin A (sc-166503; Santa Cruz Biotechnology, Santa Cruz, CA, USA) expression was evaluated by immunofluorescence and integrin β1 (sc-6622; Santa Cruz Biotechnology) was stained to identify the basement membrane. Proliferating cell nuclear antigen (PCNA, sc-7907; Santa Cruz Biotechnology) and p63 (sc-8431; Santa Cruz Biotechnology) were stained to evaluate epidermal proliferation status. For the analysis, three non-overlapping areas were digitally captured using the same magnification, illumination and exposure time. To quantify the level of protein expression, we analyzed the signal intensity using Image Pro-Plus 6.0 (MediaCybernetics Inc., Rockville, MD, USA). Finally, mRNA sequencing was used to elucidate gene expression. A more than fourfold ratio was considered a significant difference in gene expression.
Histologically, epidermal stratification was present in all of the LSE models. Interestingly, we observed that MSC-based LSEs showed more cuboidal shaped keratinocytes along the basal layer of the LSE (
Fig. 1A~C). When comparing the level of expression by image analysis, the difference of the means of signal intensity among the LSE models were statistically significant for all proteins (
Table 1). Activin A was strongly expressed in the epidermis and dermis of the MSC-based LSEs (
Fig. 1E, F) compared to fibroblast-based LSEs (
Fig. 1D). In addition, MSC-based LSEs showed stronger integrin β1 expressions with a thick basement membrane compared to the fibroblast-based LSEs (
Fig. 1G~I). These features were especially prominent in the ADSC-based LSE. The pattern of PCNA and p63 expression were very interesting in that both were low in the fibroblast-based LSE. However, the opposite pattern of expression was observed in the remaining two types of LSEs (
Fig. 1J~O). In the ADSC-based LSE, most of basal cells were positive for p63 but not PCNA. Most of basal cells, however, were negative for p63 but were positive for PCNA in the BMSC-based LSEs. Previously we reported similar patterns of PCNA and p63 expression in the ADSC-based LSE and hypothesized that these cells may not be in the state of proliferation although the potential of proliferation was maintained
7. Our new results replicated the results from previous independent experiments suggesting that the ADSC has regulatory effects on stem cell characteristics of basal keratinocytes. Furthermore, by providing an optimal niche intra-cellular environment, strong expression of integrin β1 was highly correlated with number of p63 cells. On the other hand, the BMSC-based LSE showed a completely different pattern that suggests almost all basal cells were in the state of proliferation. Among the three models, the BMSC-based model showed the thickest epidermis and highest number of PCNA positive cells that was consistent with the histological features. Different functional roles suggest these cells could be used in clinical application.
To analyze the expression of these cells, mRNA sequencing revealed that the inhibin βA gene was up-regulated (10.7-fold increase for BMSC and 8.5-fold increase for ADSC) but other genes in the activin subunits were not differentially expressed compared to fibroblasts. Kyoto Encyclopedia of Genes and Genomes pathway analysis also revealed that the gene activin A was the only up-regulated gene among the other activin subunits.
In the study, we used LSE models to investigate the effects of MSCs during skin regeneration. The results showed that epidermal proliferation and basement membrane formation were improved by the presence of these cells. This improvement can be explained by the paracrine effects of stem cells and their derivatives to secrete various factors including antioxidant mediators and cytokines
8. Among them, we postulated that activin A could be an important factor in the wound healing process. In a mouse model, activin A promoted healthy granular tissue formation, regenerated epidermal keratinocytes, and influenced keratinocyte migration
4. In hair follicles, this pathway is also related to the hair regeneration cycle
9. Previous reports and our findings suggest that activin A serves an important function for maintaining homeostasis of skin tissue.
Activin A is mainly produced in keratinocytes and the MSC
10, and we also verified that activin A was largely expressed in both epidermal and dermal layers of the LSE. It seems that activin A produced from the MSCs in the dermis affects the basal keratinocytes through paracrine effects, and contributes to epidermal proliferation and keratinocyte migration.
In summary, it was concluded that activin A may have an important role in wound healing as a mediator in dermal-epidermal interaction.
Figures and Tables
Fig. 1
Effects of the stem cells in the living skin equivalents (LSE) reconstruction. Sections of LSE were stained for H&E (A, B, and C), activin A (D, E, and F), integrin β1 (G, H and I; white arrows indicating basement membrane), PCNA (J, K, and L; yellow arrows indicating basal keratinocytes), and p63 (M, N and O; yellow arrows indicating basal keratinocytes). Original magnification: ×400 in (A~C) and ×200 in (D~O). Fb: fibroblast, ADSC: adipose-derived stem cell, BMSC: bone marrow mesenchymal stem, PCNA: proliferating cell nuclear antigen.
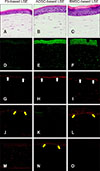
Table 1
Comparison of the level of protein expression by image analysis

|
Protein |
Signal intensity |
p-value |
Main location |
|
Fb-based LSE |
ADSC-based LSE |
BMSC-based LSE |
|
Activin A |
26.7±12.9 |
137.5±35.4 |
112.8±33.9 |
0.002 |
E |
|
Activin A |
2.7±2.0 |
16.8±6.7 |
20.9±8.1 |
0.006 |
D |
|
Integrin β1 |
31.0±10.2 |
128.0±45.6 |
70.8±28.9 |
0.002 |
BM |
|
PCNA |
16.7±7.6 |
N/D |
43.9±12.2 |
0.000 |
BL |
|
p63 |
5.1±3.2 |
35.6±10.1 |
N/D |
0.000 |
BL |
ACKNOWLEDGMENT
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant number. HI14C2040). This study was supported by the Technology Innovation Program, No. 10053020. Development of in-situ 3D bio-printing systems for wound-tailored skin regeneration funded By the Ministry of Trade, Industry & Energy (MI, Korea).
References
1. Kørbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003; 349:570–582.

2. de Kretser DM, O'Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012; 359:101–106.

3. Djouad F, Jackson WM, Bobick BE, Janjanin S, Song Y, Huang GT, et al. Activin A expression regulates multipotency of mesenchymal progenitor cells. Stem Cell Res Ther. 2010; 1:11.

4. Bamberger C, Schärer A, Antsiferova M, Tychsen B, Pankow S, Müller M, et al. Activin controls skin morphogenesis and wound repair predominantly via stromal cells and in a concentration-dependent manner via keratinocytes. Am J Pathol. 2005; 167:733–747.

5. El Ghalbzouri A, Hensbergen P, Gibbs S, Kempenaar J, van der Schors R, Ponec M. Fibroblasts facilitate re-epithelialization in wounded human skin equivalents. Lab Invest. 2004; 84:102–112.

6. Kim J, Jeong HS, Li H, Baek KJ, Kwon NS, Yun HY, et al. Effects of Cervi cornus Colla (deer antler glue) in the reconstruction of a skin equivalent model. Arch Dermatol Res. 2013; 305:85–89.

7. Huh CH, Kim SY, Cho HJ, Kim DS, Lee WH, Kwon SB, et al. Effects of mesenchymal stem cells in the reconstruction of skin equivalents. J Dermatol Sci. 2007; 46:217–220.

8. Kim SW, Lee IW, Cho HJ, Cho KH, Kim KH, Chung JH, et al. Fibroblasts and ascorbate regulate epidermalization in reconstructed human epidermis. J Dermatol Sci. 2002; 30:215–223.

9. Nakamura M, Matzuk MM, Gerstmayer B, Bosio A, Lauster R, Miyachi Y, et al. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J. 2003; 17:497–499.

10. Shao L, Frigon NL Jr, Sehy DW, Yu AL, Lofgren J, Schwall R, et al. Regulation of production of activin A in human marrow stromal cells and monocytes. Exp Hematol. 1992; 20:1235–1242.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download