Dear Editor:
Anaplastic large-cell lymphoma (ALCL) is a T cell lymphoma expressing CD30. Cutaneous ALCL can be divided into two types: primary cutaneous ALCL (PC-ALCL) that first appears in the skin and cutaneous involvement of systemic ALCL (CIS-ALCL) that first develops in lymph nodes or other organs followed by skin invasion
1. Depending on whether they express anaplastic lymphoma kinase (ALK), systemic ALCLs can be divided into two groups (ALK+ALCL and ALK−ALCL). The prognosis of each group is very different
2. Five-year survival rates of PC-ALCL, ALK+ALCL, and ALK−ALCL have been reported to be 90%, 70%~80%, and 30%~50%, respectively
12. Previous studies have identified a variety of prognostic factors, including age, stage, serum lactate dehydrogenase (LDH) level, and ALK expression in patients with systemic ALCL
123. However, prognostic factors specific for patients with CIS-ALCL are currently unknown. Therefore, the objective of this study was to analyze clinical features of CIS-ALCL and identify novel factors that could predict its prognosis. This preliminary study specifically determined the prognostic value of pretreatment white blood cell (WBC) counts. We retrospectively reviewed medical records and laboratory findings of patients diagnosed with CIS-ALCL confirmed by skin biopsy at the Dermatology Clinic of Samsung Medical Center (SMC) from January 1996 to April 2016. This study was approved by SMC Institutional Review Board (IRB no. 2016-10-042-002). We reviewed and analyzed their initial demographic information, clinical findings, and laboratory findings achieved before starting a new treatment after cutaneous lesions were histologically confirmed. Overall survival (OS) of CIS-ALCL patient was defined as the time from the confirmation of CISALCL by skin biopsy to death from any cause or last contact. Survival analysis was performed using Kaplan-Meier method. Prognostic factors associated with OS were identified by log rank test. All statistical analyses were executed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.3.1 (Vienna, Austria;
http://www.R-project.org/). A
p-value <0.05 was considered statistically significant.
Clinical findings of 12 CIS-ALCL patients were collected and analyzed (
Table 1,
2), including 7 males and 5 females with mean age of 46.5 years (range, 22~83 years). Most patients visited the clinic early after developing skin lesions, with median duration of 1 month (range, 1~36 months). Two patients were unaware of the onset time of the skin lesion. Most (91.7%) patients had multiple skin lesions. Eight (66.7%) patients had extensive (involving several noncontiguous anatomical sites) distribution. Lower extremities were involved in 7 (58.3%) cases. B-symptoms were present in 4 (33.3%) patients. In laboratory findings, LDH was elevated in 6 (50%) cases. Three (25%) cases showed leukocytosis (reference: 3.5−10.5×10
3/mm
3). Their range of WBC count was 14.86×10
3/mm
3 to 35.6×10
3/mm
3. C-reactive protein (CRP) levels were elevated in 5 of 7 patients who had CRP data. Three cases were at stage I&II. The remaining 9 cases were at stage III&IV. In immunohistochemistry, ALK was positive in 2 out of 10 patients.
Table 1
Clinical findings of patients with cutaneous involvement of systemic anaplastic large cell lymphoma

|
No. |
Sex/Age |
Duration (mo) |
Presenting skin lesions |
Extent of cutaneous involvement |
Distribution |
Extracutaneous findings |
Staging |
ALK |
B Sx. |
LDH ↑ |
leukocytosis |
CRP ↑ |
Treatment |
Outcome |
Skin Bx to outcome (mo) |
|
1 |
F/33 |
1 |
Nodule |
S, L |
LE |
LN |
IIA |
(+) |
(−) |
(+) |
(−) |
NA |
CHOP |
AW |
12 |
|
2 |
M/44 |
2 |
Nodule |
M, L |
T |
Multiple visceral metastases |
IVA |
(+) |
(−) |
(−) |
(−) |
(−) |
CHOP→Other CTx→ RT→SCT |
DWD |
7 |
|
3 |
M/34 |
1 |
Papule |
M, L |
LE |
LN, ENT lesion, Lung metastasis |
IIB |
(−) |
(+) |
(+) |
(−) |
NA |
CHOP→Other CTx→ RT→Other CTx |
DWD |
9 |
|
4 |
M/22 |
36 |
Nodule, ulcer |
M, E |
H&N, T, UE, LE |
LN |
IVA |
(−) |
(−) |
(−) |
(−) |
NA |
CHOP→Other CTx→RT |
AW |
55 |
|
5 |
F/67 |
6 |
Nodule, ulcer, swelling |
M, E |
H&N, UE |
LN, ENT lesion, Lung metastasis |
IVB |
(−) |
(+) |
(−) |
(+) |
(+) |
CHOP |
DWD |
2 |
|
6 |
M/61 |
1 |
Nodule |
M, E |
H&N, T |
LN, ENT lesion |
IIIB |
(−) |
(+) |
(+) |
(+) |
(+) |
CHOP→Other CTx→ SCT→Other CTx |
DWD |
1 |
|
7 |
M/57 |
NA |
Nodule |
M, L |
T |
LN, Lung metastasis |
IVA |
(−) |
(−) |
(+) |
(+) |
(+) |
Other CTx |
DWD |
3 |
|
8 |
M/66 |
1 |
Nodule |
M, E |
H&N, LE |
LN, Multiple visceral metastases, BM involvement |
IVA |
(−) |
(−) |
(+) |
(−) |
(+) |
CHOP→RT→Other CTx |
DWD |
2 |
|
9 |
F/35 |
1 |
Nodule |
M, E |
T, UE, LE |
LN |
IVA |
(−) |
(−) |
(−) |
(−) |
NA |
CHOP→Other CTx |
AW |
84 |
|
10 |
F/29 |
17 |
Nodule |
M, E |
H&N, UE |
LN, Multiple |
IVB |
(−) |
(+) |
(+) |
(−) |
(+) |
CHOP |
DWD |
2 |
|
11 |
M/27 |
1 |
Papule |
M, E |
H&N, LE |
LN, Tongue mass |
IIA |
ND |
(−) |
(−) |
(−) |
NA |
CHOP→RT |
AWD |
8 |
|
12 |
M/83 |
NA |
Nodule, ulcer |
M, E |
UE, LE |
LN |
IVA |
ND |
(−) |
(−) |
(−) |
(−) |
Symptomatic Tx |
DWD |
8 |

Table 2
Survival outcome of patients with cutaneous involvement of systemic anaplastic large-cell lymphoma
|
Variables in CIS-ALCL |
Number (%) |
Median OS after the confirmation of skin involvement (mo) |
p-value*
|
1-year survival rate, % |
|
Multiplicity |
Multiple |
11 (91.7) |
7.0 |
0.254 |
24.2 |
|
Solitary |
1 (8.3) |
NE |
NA |
|
Extent |
Extensive |
8 (66.7) |
5.0 |
0.888 |
37.5 |
|
Localized |
4 (33.3) |
8.0 |
25.0 |
|
Distribution |
LE |
7 (58.3) |
NE |
0.003†
|
53.6 |
|
Non-LE |
5 (41.7) |
2.0 |
0.0 |
|
B-symptoms |
Positive |
4 (33.3) |
2.0 |
0.054 |
0.0 |
|
Negative |
8 (66.7) |
8.0 |
50.0 |
|
LDH elevation |
Positive |
6 (50.0) |
2.5 |
0.215 |
16.7 |
|
Negative |
6 (50.0) |
8.0 |
50.0 |
|
Leukocytosis |
Positive |
3 (25.0) |
2.0 |
0.010†
|
0.0 |
|
Negative |
9 (75.0) |
9.0 |
41.7 |
|
CRP elevation |
Positive |
5 (71.4) |
2.0 |
0.032†
|
0.0 |
|
Negative |
2 (28.6) |
7.5 |
0.0 |
|
Staging |
III, IV |
9 (75.0) |
3.0 |
0.144 |
22.2 |
|
I, II |
3 (25.0) |
9.0 |
50.0 |
|
ALK expression |
Positive |
2 (20.0) |
7.0 |
0.438 |
50.0 |
|
Negative |
8 (80.0) |
2.5 |
25.0 |

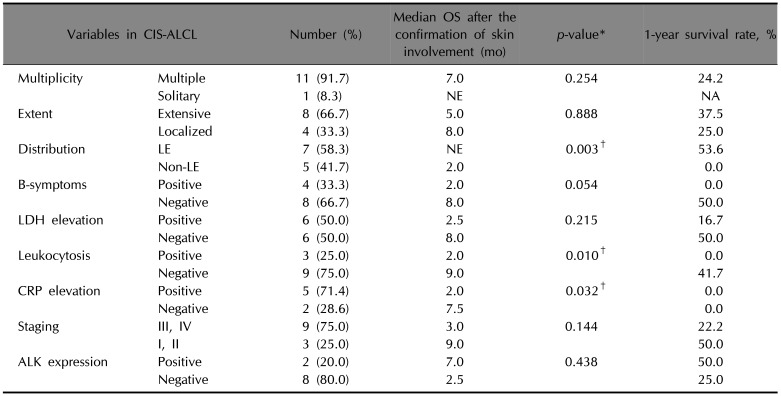
Leukocytosis predicted worse survival outcome (p=0.010). Median OS (MOS) was 9 months in patients without leukocytosis and 2 months in patients with leukocytosis. The 1-year survival rate (1YSR) was 41.7% in patients without leukocytosis and 0% in patients with leukocytosis. Cases without CRP elevation showed MOS of 7.5 months while cases with CRP elevation showed MOS of 2 months (p=0.032). In addition, patients with involvement of lower extremities showed better survival outcome than patients without involvement of lower extremities (p=0.003). Multiplicity, extent, B-symptoms, LDH elevation, staging, or ALK expression did not affect survival.
Several studies have suggested that older age, high LDH level, poor performance status, B-symptoms, advanced stage, extranodal involvement, and the absence of ALK expression are unfavorable prognostic factors for systemic ALCL
145. Similar to previous reports on systemic ALCL, the current study showed worse MOS and 1YSR in patients with B-symptoms, LDH elevation, advanced stage, and without ALK expression, although statistical significance was not shown.
Interestingly, our analysis suggested predictive value of WBC count and CRP level. High WBC count has been reported to be a poor prognostic factor for other diseases such as Hodgkin's lymphoma, mantle cell lymphoma, cervical cancer, non-small cell lung cancer, melanoma, and breast cancer
6. No previous study has confirmed the association between leukocytosis and the survival rate of CIS-ALCL patients. However, some studies have reported that cases of systemic ALCL with leukocytosis show poor clinical outcomes
789. In a case series reported by Chang et al.
7, the median value of WBC count was 22.7×10
3/mm
3 (range: 15.3–112.9×10
3/mm
3). Four of five patients died within 3.5 weeks
7. The authors suggested that the release of cytokines such as G-CSF and tumor necrosis factor-α might be associated with leukocytosis of ALCL
7. In another case report, ALCL cases showing extreme neutrophilia have been reported and prognostic value of interleukin-17 has been suggested
10. Based on these case reports, the need to study the relationship between leukocytosis and prognosis of ALCL has increased.
Although the current study suggested that WBC count might be a prognostic factor for clinical outcome, the number of patients used in this study with leukocytosis was small. This might have caused bias in results. The most important confounding factor to consider in the causal relation between leukocytosis and survival outcome is infection. Therefore, we reviewed the presence of evidence of infection when CIS-ALCL was identified in 12 cases. Results of serology and culture (including blood, urine, and wound swab) were negative in all cases except one which showed positivity for anti-hepatitis C virus antibodies. However, this case showed no elevation of WBC count. For the three cases showing leukocytosis, there was no evidence of infection. Therefore, the effect of infection was considered to be negligible in this study.
In conclusion, results of this preliminary study suggested that elevation of WBC count might be a poor prognostic factor in CIS-ALCL patients. However, since our sample size was small, it was difficult to exclude the effect of unmeasured confounders or confirm statistical significance. Therefore, a multi-center study with a larger number of samples is needed to confirm our results in the near future.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download