Abstract
Although a few reports have noted the concurrent presentation of morphea and vitiligo at distinctly separate sites in the same patient, it is extremely rare that these two conditions occur at the same sites in a patient. We report the case of a 10-year-old Korean girl with morphea and vitiligo and those lesions occurred at the same sites and progressed simultaneously. An autoimmunity and a cutaneous mosaicism was considered to be involved in such an unique presentation as the pathogenesis is concerned.
Morphea, also called localized scleroderma is a rare fibrosing disorder affecting the skin and underlying tissues. Segmental vitiligo is a distinct form of vitiligo, a common acquired pigmentation disorder, with dermatomal distribution or distribution of lesions along the Blaschko's lines. A few reports have previously noted the concurrent presentation of morphea and vitiligo at distinctly separate sites in the same patient12345678. However, it is extremely rare that these two diseases occur at the same site in a patient.
A 10-year-old Korean girl presented with multiple ill-defined hypopigmented patches on her left arm for 2 years (Fig. 1A). The lesions had an indurated, atrophic, and shiny area at the center. They gradually continued to increase in size over 2 years. Before presenting to outpatient clinic of Department of Dermatology, SMG-SNU Boramae Medical Center, she had received topical corticosteroids, excimer laser therapy, and Oriental remedies without any improvement. There was no history of trauma or injury to the site.
Physical examination revealed three depigmented patches with small satellite lesions on the left upper arm (Fig. 1A). The lesions were distributed along the T1 dermatome, and showed a central sclerotic area (Fig. 1B). In Wood's lamp examination, the lesions were accentuated compared to the peripheral uninvolved skin. The patient did not present with sclerodactyly, Raynaud's phenomenon, nailfold capillary changes, or signs of any systemic involvement, and a general physical examination was unremarkable.
Punch biopsies performed at both sites, the hypopigmented area and normal uninvolved periphery revealed that the depigmented lesions demonstrated decreased basal pigmentation, mainly reduced numbers of melanocytes, as well as degenerated thickened dermal collagen bundles with focal dense lymphocytic infiltration, compared to adjacent normal skin (Fig. 2A, B). A biopsy from the normal periphery showed only mild superficial perivascular lymphocytic infiltration (Fig. 2C). Melan-A/MART-1 and S-100 positive melanocytes were noted to be decreased in the depigmented lesions compared to the normal periphery (Fig. 2D~G). Based on clinical and histopathological findings, she was diagnosed as circumscribed morphea accompanied by segmental vitiligo.
She was administered intra-lesional triamcinolone injections four times, and topical tacrolimus 0.1% ointment was prescribed to be applied twice daily. After six months of treatment, the lesions showed substantial improvement with repigmentation (Fig. 3).
We received the patient's consent about publishing all photographic materials.
A concurrent presentation of vitiligo and morphea has been reported, and such an association is noted to be more frequent than can be attributed to mere coincidence12345678. Based on some aspects of our case, we can propose that a definite relationship exists between the two diseases. In our patient, the two conditions progressed and improved simultaneously. Wood's lamp exam and results of specific stain (Melan-A/MART-1 and S-100 stain) favor vitiligo than a simple pigmentary change in morphea or lichen sclerosus. Also, the probability of scar tissue is low in that there was no traumatic history and previous performed oriental remedy was an oral medication that could not give trauma to the lesion. Furthermore, our case differed from previous reports in that the lesions of the two diseases were almost overlapping, and such an unique presentation supports our proposal that a definite association exists between the two diseases and their concurrent presentation is not a coincidence.
Various hypotheses have been proposed to explain the etiology of these two diseases; cutaneous mosaicism and autoimmunity are common to both conditions and may explain the presentation in our patient.
Interestingly, the lesions were distributed along Blaschko's lines in our case. Cutaneous mosaicism may explain the segmental distribution and linkage between the two diseases. A possible explanation could be that a clone of vulnerable cells is formed during embryogenesis and exposure of these cells to an appropriate trigger such as an autoimmune attack may result in the development of morphea and vitiligo9.
Morphea is considered to be an autoimmune disease based on various recent studies, and is associated with various other autoimmune diseases, that is, primary biliary cirrhosis10, myasthenia gravis11, Hashimoto's thyroiditis56, autoimmune thrombocytopenic purpura12, dermatomyositis, pemphigus13 and multiple autoimmune syndrome8. Moreover, circulating cytokines and soluble receptors are known to increase in patients with morphea; including interleukin-2 (IL-2), IL-4, IL-6, IL-13, soluble interleukin-2 (sIL-2) receptor and sIL-6, soluble CD4 and CD8, among others51314. Additionally, there is a higher incidence of autoantibodies such as anti-nuclear antibody (ANA) and anti-single-stranded DNA (ssDNA) in the serum of patients with morphea, and their relatives.51314. Vitiligo is also associated with various autoimmune diseases such as diabetes1516, pernicious anemia17, Addison's disease4, thyroid disease18, and alopecia areata4. Various organ-specific autoantibodies have been detected in the serum of patients with vitiligo515. In addition, the particular alleles of the multilocus major histocompatibility complex (MHC) are associated with susceptibility to vitiligo51920. Based on these findings, an autoimmune mechanism seems to be playing an important role in the concurrent presentation of the two diseases. This association therefore suggests that the regular screening for development of autoimmune diseases may be imperative in these patients.
Nevertheless, pathogenesis of morphea and vitiligo still eludes our understanding, the reported association between morphea and vitiligo, as was seen in our patient, does provide a better understanding of the pathogenesis of the two conditions. Further studies would be needed to elucidate whether the two conditions are definitively associated, and to determine a hypothesis that can explain the concurrence of the two diseases.
Figures and Tables
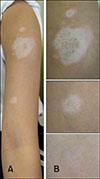 | Fig. 1(A) Multiple hypopigmented patches with small satellite lesions on the left upper arm, distributed along the T1 dermatome. (B) Indurated, atrophic, shiny areas at the center of hypopigmented patches. |
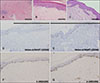 | Fig. 2(A) Biopsy from the depigmented lesion (H&E, ×100). Degenerated thickned dermal collagen bundles with focal dense lymphocytic infiltration. (B and C) Biopsy from the depigmented lesion (H&E, ×400) (B) showed a decresed basal pigmentation compared to adjacent normal peripheray (H&E, ×400) (C). Mild superficial perivascular lymphocytic infiltration was observed in the normal periphery (C). (D and E) Biopsy from the depigmented lesion (Melan-A/MART-1, ×200) (D) showed decreased Melan A positive melanocytes compared to adjacent normal periphery (Melan-A/MART-1, ×200) (E). (F and G) Biopsy from the depigmented lesion (S-100, ×200) (F) showed decreased S-100 positive melanocytes compared to adjacent normal periphery (S-100, ×200) (G). |
References
1. Bonifati C, Impara G, Morrone A, Pietrangeli A, Carducci M. Simultaneous occurrence of linear scleroderma and homolateral segmental vitiligo. J Eur Acad Dermatol Venereol. 2006; 20:63–65.

2. Yadav P, Garg T, Chander R, Nangia A. Segmental vitiligo with segmental morphea: an autoimmune link? Indian Dermatol Online J. 2014; 5:Suppl 1. S23–S25.

3. Finkelstein E, Amichai B, Metzker A. Coexistence of vitiligo and morphea: a case report and review of the literature. J Dermatol. 1995; 22:351–353.

5. Yorulmaz A, Kilic S, Artuz F, Kahraman E. Concomitant appearance of morphea and vitiligo in a patient with autoimmune thyroiditis. Postepy Dermatol Alergol. 2016; 33:314–316.

6. Dervis E, Acbay O, Barut G, Karaoglu A, Ersoy L. Association of vitiligo, morphea, and Hashimoto's thyroiditis. Int J Dermatol. 2004; 43:236–237.

7. Soylu S, Gul U, Gönül M, Kilic A, Cakmak SK, Demiriz M. An uncommon presentation of the co-existence of morphea and vitiligo in a patient with chronic hepatitis B virus infection: is there a possible association with autoimmunity? Am J Clin Dermatol. 2009; 10:336–338.

8. Bonilla-Abadia F, Muñoz-Buitrón E, Ochoa CD, Carrascal E, Cañas CA. A rare association of localized scleroderma type morphea, vitiligo, autoimmune hypothyroidism, pneumonitis, autoimmune thrombocytopenic purpura and central nervous system vasculitis. Case report. BMC Res Notes. 2012; 5:689.

9. Soma Y, Fujimoto M. Frontoparietal scleroderma (en coup de sabre) following Blaschko's lines. J Am Acad Dermatol. 1998; 38:366–368.

10. Gonzalez-Lopez MA, Drake M, Gonzalez-Vela MC, Armesto S, Llaca HF, Val-Bernal JF. Generalized morphea and primary biliary cirrhosis coexisting in a male patient. J Dermatol. 2006; 33:709–713.

11. Parra V, Driban N, Bassotti A. Localized morphea and myasthenia gravis. J Am Acad Dermatol. 2003; 49:E12.

12. Neucks SH, Moore TL, Lichtenstein JR, Baldassare AR, Weiss TD, Zuckner J. Localized scleroderma and idiopathic thrombocytopenia. J Rheumatol. 1980; 7:741–744.
13. Arif T, Hassan I, Nisa N. Morphea and vitiligo-A very uncommon association. Our Dermatol Online. 2015; 6:232–234.

14. Takehara K, Sato S. Localized scleroderma is an autoimmune disorder. Rheumatology. 2005; 44:274–279.

15. Cunliffe WJ, Hall R, Newell DJ, Stevenson CJ. Vitiligo, thyroid disease and autoimmunity. Br J Dermatol. 1968; 80:135–139.

17. Grunnet I, Howitz J, Reymann F, Schwartz M. Vitiligo and pernicious anemia. Arch Dermatol. 1970; 101:82–85.

18. Bor S, Feiwel M, Chanarin I. Vitiligo and its aetiological relationship to organ-specific autoimmune disease. Br J Dermatol. 1969; 81:83–88.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download