Abstract
Background
Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a flavonoid known to have a wide range of pharmacological activities. The 3-OH group in flavonoids has been reported to determine antioxidant activities.
Objective
We tested whether kaempferol can affect the expression of integrins and the stem cell fate of interfollicular epidermal stem cells.
Methods
Skin equivalent (SE) models were constructed, and the expression levels of stem cell markers and basement membrane-related antigens were tested. The immunohistochemical staining patterns of integrins, p63, and proliferating cell nuclear antigen (PCNA) were compared between kaempferol- and apigenin-treated SE models. Reverse transcription-polymerase chain reaction (RT-PCR) was used to evaluate the mRNA expression of integrins.
Results
Kaempferol increased the thickness of the epidermis when added to prepare SEs. In addition, the basal cells of kaempferol- treated SEs appeared more columnar. In the immunohistological study, the expression of integrins α6 and β1 and the numbers of p63- and PCNA-positive cells were markedly higher in the kaempferol-treated model. However, apigenin showed no effects on the formation of three-dimensional skin models. RT-PCR analysis also confirmed that kaempferol increased the expression of integrin α6 and integrin β1.
Conclusion
Our findings indicated that kaempferol can increase the proliferative potential of basal epidermal cells by modulating the basement membrane. In other words, kaempferol can affect the fate of interfollicular epidermal stem cells by increasing the expression of both integrins α6 and β1. These effects, in particular, might be ascribed to the 3-OH group of kaempferol.
Kaempferol, a type of natural flavonoid, has been isolated from Delphinium, witch hazel, grapefruit, apples, tea, broccoli, and other plant sources1. Previous epidemiological studies reported a positive correlation between the taking foods containing kaempferol and a reduced risk of some disorders such as cardiovascular diseases and malignant tumors2. In addition, several preclinical studies have shown that kaempferol and its derivatives have a wide range of pharmacological activities, such as antioxidant, anti-inflammatory, antimicrobial, antidiabetic, analgesic, and anti-allergic activities23456.
Aging is a complex biological phenomenon affecting the different components of the skin7. In particular, photoaged skin represents prominent histological changes in the collagenous extracellular matrix (ECM) of connective tissue8. Because kaempferol has antioxidant and anti-inflammatory activities, it is expected that kaempferol can slow the skin aging process and ameliorate aging-related skin problems. The skin is a multi-layered organ composed of the epidermis and dermis, which are separated by the basement membrane. The basement membrane of sun-exposed skin has been reported to become damaged and partly disrupted compared with that of sun-protected skin9. However, the aging changes in the basement membrane are not fully understood at present. The epidermis is a continually renewing tissue composed of keratinocytes, which can be divided into basal stem cells, transit-amplifying cells, and post-mitotic differentiating cells10. Recently, cell-ECM interactions—which control epidermal stem cell fate—have been implicated in influencing normal homeostasis, aging, and wound-healing processes of the skin11. Therefore, strategies for manipulating cell-ECM interactions might constitute the keystone for controlling skin diseases or designing antiaging approaches in future.
In this study, the effects of kaempferol on cell-ECM interactions were tested on skin equivalent (SE) models. The 3-OH group of flavonoids has been reported to be important in antioxidant activities12. Because the formula of kaempferol is 3,4′,5,7-tetrahydroxyflavone, the effect of apigenin (4′,5,7-trihydroxyflavone) was simultaneously tested owing to the structural similarities (except for the 3-OH group) of both compounds (Fig. 1A, B).
To measure the antioxidant effects, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was used. DPPH is a stable free radical molecule commonly used in antioxidant assays. Three concentrations were used, and each sample of stock solution (2 µl of 100X) was added to 80 µl of 0.25 mM DPPH and 118 µl of 70% ethanol to produce a final DPPH concentration of 0.1 mM. The mixture was vigorously shaken and left to stand in the dark, and its absorbance was measured at 517 nm using an ELISA reader after 30 minutes (TECAN, Salzburg, Austria). Ascorbic acid was used as the control.
Human keratinocytes were collected from foreskins obtained during child circumcision in our hospital. Skin specimens were processed and cultured according to the method of Rheinwald and Green13 with some modification, as we mentioned in the previous paper14. Fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM, LM001-05; WelGENE, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT, USA).
The cultured keratinocytes and fibroblasts were seeded into 24-well plates. After 24 hours of growth factor starvation or serum starvation, cells were incubated with kaempferol (up to 20 µM) or apigenin (up to 10 µM) for 24 hours at 37℃. MTT solution (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; 100 µl of 5 mg/ml concentration) was then added, after which the plates were incubated for an additional 4 hours. The optical density was measured at 540 nm using an ELISA reader (TECAN).
SEs were constructed following our previous method15. A low concentration of epidermal growth factor (EGF, 1 ng/ml; Sigma, St. Louis, MO, USA) was also added during the submerged culture, and a higher concentration of EGF (10 ng/ml) was added during the air-liquid interface culture. Both kaempferol and apigenin were added during air-exposure period for 10 days. The medium was changed 3 times per week, and all experiments were repeated at least twice under the same conditions.
After 2 weeks, SEs were fixed in Carnoy's solution for 30 minutes and processed for conventional paraffin embedment. Sections (4~6-µm-thick) were then prepared. For morphological observations, hematoxylin and eosin (H&E) staining was used. For immunohistochemical analysis, sections were processed using the avidin-biotin-peroxidase complex technique (DAKO, Glostrup, Denmark). Antibodies against p63 (#sc-8431), integrin α6 (#sc-6597), and integrin β1 (#sc-9970) were obtained from Santa Cruz Biotechnology, Inc.; proliferating cell nuclear antigen (PCNA) (#M0879), from DAKO; and involucrin (#I-9018), from Sigma Chemical Co.
Keratinocytes (100,000) were seeded in 6-well plates. The cells were cultured in media containing kaempferol (0, 5, 10, 20 µM) for 72 hours. Total RNA was isolated using the AllPrep DNA/RNA/Protein Mini Kit (80004; Qiagen, Valencia, CA, USA). The quality and amount of RNA were assessed using the Experion RNA StdSens analysis kit (700-7104; Bio-Rad, Hercules, CA, USA) by the Experion automated electrophoresis system (700-7001; Bio-Rad). For the quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) of human integrin α6 (ITGA6) and integrin β1 (ITGB1), 1 µg of total RNA was reverse transcribed into cDNA (ImProm-II Reverse Transcription System, A3800; Promega, Madison, WI, USA) with the Oligo (dT) 15 primer following the manufacturer's instructions. Real-time PCR was performed with TaqMan Gene Expression Master Mix (4369016; Applied Biosystems, Foster City, CA, USA) and the target gene primers ITGA6 (4331182, Hs01041011_m1; Applied Biosystems) and ITGB1 (4331182, Hs00559595_m1; Applied Biosystems). The housekeeping gene GAPDH (43352934E, Applied Biosystems) was used as an endogenous control.
The optical density was measured at different time points. Result obtained at 30 minutes after incubation was recorded. Kaempferol showed effective antioxidant activity from 0.025 mM concentration onwards. However, apigenin was not effective up to 2.5 mM concentration (Fig. 1C).
For the culture of SEs, cytotoxicity was tested. Results showed that kaempferol was not toxic to keratinocytes up to a concentration of 20 µM (Fig. 2A). For fibroblasts, similar results were obtained (Fig. 2B). Accordingly, 10 µM of kaempferol was selected for further experiments. Apigenin was not found to be toxic to either keratinocytes or fibroblasts up to a concentration of 10 µM (Fig. 2C, D). Thus, 5 µM apigenin was chosen for further experiments.
H&E staining revealed typical morphological features with regular stratification of the epidermis. Especially in SEs treated with kaempferol, the basal and suprabasal layers were more compact and appeared more columnar. Epidermal thickness was also higher in the SEs treated with kaempferol (SE/kpf model) compared to the control and the SEs treated with apigenin (SE/ap model) (Fig. 3A~C).
Integrin α6 is a major component of the hemidesmosome, through which the cell adheres to the basement membrane. Integrin β1 is expressed in all basal keratinocytes and is required to maintain keratinocytes in an undifferentiated state16. Dense staining of integrins α6 and β1 was observed in the SE/kpf model (Fig. 3F, J). However, both integrins stained weakly along the dermo-epidermal junction in the control model (Fig. 3E, I). Likewise, staining was also weak in the SE/ap model (Fig. 3G, K).
We then examined the expression of p63 as a potential marker of stem cells. A significantly higher number of p63-positive cells were observed in the SE/kpf model compared to the control or SE/ap model (Fig. 4A~C). PCNA, which is a cofactor of DNA polymerase, has an important role on the cell cycle progression into the S-phase. Therefore, the number of PCNA-positive cells was also counted. More PCNA-positive cells were observed in kaempferol-treated SEs compared to the control and SE/ap models (Fig. 4E~G). The expression of involucrin, which is a protein precursor of the epidermal cornified envelope, was compared in each group. In normal skin, involucrin expression is sharply confined to the upper spinous and granular cell layers17. Our findings showed that areas of cells without involucrin expression significantly increased by kaempferol treatment compared to other models (Fig. 4I~K). Because involucrin is a marker of differentiation, these findings suggest that kaempferol delayed the differentiation of keratinocytes.
Cultured normal human keratinocytes were treated with increasing concentrations of kaempferol, and mRNA was extracted. RT-PCR analysis showed that kaempferol increased the expression of both integrins α6 and β1 in a dose-dependent manner (Fig. 5).
Kaempferol is a natural flavonol found in a variety of plants and plant-derived foods. It is slightly soluble in water and highly soluble in hot ethanol, ethers, and DMSO. Its antioxidant properties are well known, and many studies suggest that consuming kaempferol might reduce the risk of various cancers. Therefore, kaempferol is currently under consideration as a possible anticancer agent3. Since its molecular weight is small enough to penetrate the skin, its use as a method of directly applying it to the skin is also considered. In the present study, when we tested the antioxidant activity of kaempferol by the DPPH assay, it proved to be highly effective. Apigenin is a natural product belonging to the flavone class, which comprises aglycones of several naturally occurring glycosides18. The only structural difference between kaempferol and apigenin is the presence and absence, respectively, of 3-OH. However, the DPPH assay showed that apigenin did not have antioxidant activities in our tested condition. With these results, we suggest that presence or absence of 3-OH is important in stem cell activating effects in skin equivalent. However, apigenin itself may have these effects regardless of absence of 3-OH, which need further research.
Antioxidant and anti-inflammatory activities of kaempferol provide evidence suggesting its role in skin health. However, the effects of kaempferol on skin stem cells have not been elucidated thus far. To analyze the effects of kaempferol, a three-dimensional skin model was treated with kaempferol and apigenin. After testing the cytotoxicity, SEs were constructed and treated with kaempferol. H&E staining showed that kaempferol treatment increased the thickness of the epidermis. Furthermore, the shape of basal keratinocytes appeared more columnar compared to those of the control or apigenin-treated models. Because the columnar shape of basal cells suggests healthy cells, as opposed to flattened cells, these findings suggest that treatment with kaempferol increased the proliferative potential of basal epidermal cells.
We next performed immunohistochemical staining to check the basement membrane status. Epidermal stem cells are believed to be located in the bulge area of hair follicles, the basal layer of interfollicular epidermis, and the base of sebaceous glands. The self-renewal of stem cells is driven intrinsically by gene expression, and it is modulated through interaction with extrinsic cues from the environment19. Thus, modulation of the stem-cell niche is important for the self-renewal and multipotency of stem cells. Integrins are important for the maintenance of the stem-cell niche and in signal transduction during skin development. To verify integrin expression, sections of SEs were stained for integrin α6 and integrin β1. Results showed that the staining of integrin in SEs was markedly increased by the addition of kaempferol. Meanwhile, apigenin had no effects on integrin expression.
Furthermore, the number of p63-positive cells increased by kaempferol treatment, and the number of PCNA-positive cells increased. p63 is a transcription factor that plays a central role in epithelial development, and it has been reported to regulate basal epithelial cell adhesion and survival. PCNA is present throughout the cell cycle in proliferating cells, and PCNA expression is mainly observed in the basal layer of normal skin20. These findings all suggest that kaempferol increased the proliferative potential of basal epidermal cells.
In vitro SE skin models are used to conduct experiments on processes involving the skin21. In this study, the effects of kaempferol and apigenin were tested using SE models. The effect of apigenin was not as satisfactory as that of kaempferol in any of the experiments. Despite their structural similarity, the effects of kaempferol on skin cells seemed to be unique among the flavonoid derivatives. Skin aging is induced by impaired stem cell mobilization or by a reduction in the number of stem cells that can respond to proliferative signals. We recently reported that oligosaccharides such as hyaluronic acid, the tripeptide Gly-His-Lys, and various antioxidants can affect the proliferative potential of epidermal basal cells by providing a favorable microenvironment22. In this study, the effects of kaempferol on the basement membrane and the subsequent effects on skin stem cells were clearly demonstrated.
Epidermal homeostasis depends on a balance between the renewal and differentiation of stem cells, and this is regulated by extrinsic signals from the ECM. Because immunohistochemical staining showed integrin expression to have been increased by kaempferol treatment, we tested whether kaempferol can increase the expression of both integrins in cultured normal human keratinocytes. RT-PCR also clearly showed that integrin expression was increased by kaempferol treatment. To our knowledge, this is the first report demonstrating that kaempferol might increase the proliferative potential of keratinocytes by regulating ECM proteins such as integrin α6 and integrin β1. The exact interaction mechanisms of kaempferol with the ECM and epidermal stem cells warrant further in-depth investigation.
Figures and Tables
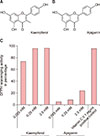 | Fig. 1The molecular structures of kaempferol and apigenin. Their structures are the same except for the presence and absence, respectively, of the 3-OH functional group (A, B). Kaempferol demonstrated satisfactory antioxidant activity from a concentration of 0.025 mM onwards in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, while apigenin did not (C). |
 | Fig. 2Cytotoxicity of kaempferol (A, B) and apigenin (C, D) to cultured normal keratinocytes and fibroblasts. Cells were treated as described in the “materials and methods.” The y-axis represents the relative cell viability following the MTT assay. The values shown are the means±standard deviation of triplicate wells. |
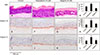 | Fig. 3The effects of kaempferol and apigenin on skin equivalent reconstruction and integrin expression. Sections of skin equivalents were stained for hematoxylin and eosin (H&E) (A~C), integrin α6 (E~G), and integrin β1 (I~K). Stained sections were analyzed with an image analysis program (D, H, L). Experiments were performed twice with the same cells; representative results are shown (original magnification: ×400, **p<0.01 in comparison with the control group). |
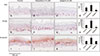 | Fig. 4Immunohistochemical staining for proliferative markers and involucrin. Sections were stained for p63 (A~C), proliferating cell nuclear antigen (PCNA) (E~G), and involucrin (I~K), respectively. The expression levels were analyzed with an image analysis program (D, H, L). Experiments were performed twice with the same cells; representative results are shown (original magnification: ×400, *p<0.05; **p<0.01 in comparison with the control group). |
ACKNOWLEDGMENT
This study was supported by grant number 16-2015-008 from the Seoul National University Bundang Hospital Research Fund; a grant (HN14C0094) from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea; and the Technology Innovation Program, 10053020, Development of In Situ 3D Bioprinting System for Wound-Tailored Skin Regeneration funded by the Ministry of Trade, Industry & Energy (MI, Korea).
References
1. Mustafa RA, Abdul Hamid A, Mohamed S, Bakar FA. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J Food Sci. 2010; 75:C28–C35.

2. Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011; 11:298–344.

3. Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013; 138:2099–2107.

4. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52:673–751.
5. Luo C, Yang H, Tang C, Yao G, Kong L, He H, et al. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol. 2015; 28:744–750.

6. Kim M, Lim SJ, Kang SW, Um BH, Nho CW. Aceriphyllum rossii extract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. J Agric Food Chem. 2014; 62:3750–3758.

7. El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002; 11:398–405.

8. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997; 337:1419–1428.

9. Amano S. Possible involvement of basement membrane damage in skin photoaging. J Investig Dermatol Symp Proc. 2009; 14:2–7.

10. Kaur P, Li A. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol. 2000; 114:413–420.

11. Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011; 3:pii: a005124.

12. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996; 20:933–956.

13. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6:331–343.

14. Choi HR, Park SH, Choi JW, Kim DS, Park KC. A simple assay method for melanosome transfer. Ann Dermatol. 2012; 24:90–93.

15. Kim DS, Cho HJ, Choi HR, Kwon SB, Park KC. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell Mol Life Sci. 2004; 61:2774–2781.

17. Li ER, Owens DM, Djian P, Watt FM. Expression of involucrin in normal, hyperproliferative and neoplastic mouse keratinocytes. Exp Dermatol. 2000; 9:431–438.

18. Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001; 49:3106–3112.

19. Choi HR, Byun SY, Kwon SH, Park KC. Niche interactions in epidermal stem cells. World J Stem Cells. 2015; 7:495–501.

20. Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987; 326:515–517.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download