Abstract
Background
There are few pharmacologic options to reduce erythema and flushing in patients with recalcitrant erythematotelangiectatic rosacea (ETR). We previously reported two cases of refractory flushing and erythema of rosacea that were successfully treated with intradermal botulinum toxin injection, and additional research is needed to prove the efficacy and safety of this treatment.
Objective
To report the efficacy and safety of botulinum toxin injection as an aid in persistent erythema of rosacea patients.
Methods
A total of 20 Korean patients with recalcitrant ETR were enrolled to receive treatment by injection of botulinum toxin. Patients received one treatment of intradermal botulinum toxin injection and were assessed 1, 2, 4, and 8 weeks after treatment. The severity of erythema and telangiectasia was investigated by a non-treating physician, and the Erythema Index (EI) was assessed by mexameter at each visit. Patient satisfaction and any adverse events were also assessed at each visit.
Results
17 patients completed all follow-up visits and were included in the analysis. Intradermal injection of botulinum toxin significantly reduced erythema severity and EI in ETR patients. Patients reported a satisfaction score of 2.94±0.56 at 8 weeks after treatment. Except for three patients who discontinued the study early due to inconvenience of facial muscle paralysis, 17 patients participating in the final analysis did not report side effects except injection pain at the time of the procedure.
Rosacea is a common chronic skin condition that displays clinical variability with erythema, telangiectasia, papules, pustules, and phymata. The etiology of this disease is also multifactorial, and neurovascular dysregulation, inflammation, and hyperseborrhea all contribute to disease pathogenesis.
A few recent studies have shown confusing results regarding the efficacy of botulinum toxin (BTX) injections for erythema, flushing, and telangiectasia of rosacea. Schwab et al.1 have shown that vascular dysregulation plays a role in the development of rosacea, and BTX might stabilize vascular hyperactivity2. A study of 25 subjects with erythematotelangiectatic rosacea (ETR) indicated that intradermal injection of BTX appeared effective and safe for treatment of facial erythema of rosacea3, and another study showed that BTX reduced sebaceous gland hyperactivity and pore size4. However, there was an ineffective split-face study using BTX for improvement of facial flushing5.
Meanwhile, the flushed and telangiectatic face of rosacea is often accompanied by stinging and burning, signs of nerve activation. According to recent studies, researchers have found a potential connection between the redness, stinging, and nervous system of ETR subjects. BTX, a well-known potent neurotoxin, blocks acetylcholine (ACh) release from nerve terminals, which therefore leads to cessation of somatic motor and parasympathetic transmission. Recently, it has been found that BTX also interferes with sensory transmission by reduction of other neurotransmitters (e.g., substance P [SP], calcitonin gene-related peptide [CGRP], pituitary adenylate cyclase-activating polypeptide [PACAP]), and transient receptor potential channel vanilloid family member 1 (TRPV1) receptors678. There is increasing evidence that these neurovascular components are also involved in the pathogenesis of rosacea, and BTX may help relieve clinical symptoms by inhibiting these factors.
This study was conducted to investigate the efficacy and safety of BTX A on both cheeks of 20 patients who had persistent and recalcitrant ETR.
Twenty Korean volunteers were enrolled after being approved by the Institutional Review Board of Chung-Ang University Hospital (IRB no. C2014040 [1236]). The patients were diagnosed by standard guidelines of the National Rosacea Society Expert Committee. Their severities of erythema and telangiectasia were ≥1, and patients whose symptoms lasted longer than three months despite more than two conventional treatments were enrolled. Mandatory wash-out periods (six months for oral isotretinoin and one month for any oral medication or treatment that could affect facial erythema during one month prior to the study) were required if patients were receiving prescription medications for rosacea or acne. Gentle skin cleasner and moisturizer were provided to the patients. During the study, any other personal skin care products are inhibited. Written informed consent was obtained from all participants after the risks and benefits of the procedure were explained in detail. Also, we received the patient's consent form about publishing all photographic materials.
Digital photographs were compared to photos at each follow-up throughout the study. After initial assessment, a lidocaine-based topical anesthetic (5% EMLA cream; Astra-Zeneca, Södertälje, Sweden) was applied to the face for 1 hour and then washed off. Each vial of onabotulinumtoxin A, which contained 50 U of Clostridium BTX type A with human serum albumin and sodium chloride (Meditoxin; Meditox, Seoul, Korea), was reconstituted with 2.5 ml of sterile saline to achieve a concentration of 2 U/0.1 ml. A total of 20 units of BTX was used for each patient, and the injection points were staggered 1 cm apart to cover the erythematous lesions of both cheeks using a 30-gauge insulin syringe. Soft gauze pressure was applied to prevent bleeding, and possible acute adverse reaction was monitored for 30 minutes after injection. The severity of erythema and telangiectasia was evaluated by a non-treating investigator on a 4-point scale (0=normal, 1=mild, 2=moderate, and 3=severe)9. The erythema index (EI) was checked by mexameter MX18 (Courage-Khazaka electronic GmbH, Cologne, Germany) assessment at baseline and 1, 2, 4, and 8 weeks after treatment. Three repetitive measurements on each side of the cheek (at the point where midpupillary line and vertical line from the nasal tip meet) were performed at baseline and follow-up visits, and the mean value of the measurements was used as the EI in the analysis. Patient satisfaction was also evaluated on a 5-point scale (0=very poor, 1=poor, 2=moderate, 3=good, and 4=very good).
R language version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and T&F program ver. 2.0 (YooJin BioSoft, Goyang, Korea) were used for all statistical analyses. Data were expressed as mean±standard deviation for continuous variables. For categorical variables, data was expressed as sample number and percentage, N (%). For normally distributed variables, paired sample t-test was used if the mean difference between paired sets of observations was zero. For non-normally distributed variables, the Wilcoxon signed rank test was used. Paired sets were defined as measurements at baseline and each time point. Repeated measures ANOVA was performed to test the mean difference among repeatedly measured variables measured at baseline and each time point. If the assumption of sphericity was violated, the p-value was adjusted using the Greenhouse-Geiser method.
A total of 20 patients (18 women and 2 men) with ETR participated in the present study. The age distribution was 20~56 years, and the mean age was 35.95±11.56 years. A total of 17 subjects completed the 5 visits, and the compliance rate was 85%. Representative cases of clinical photographs are shown in Fig. 1.
The mean EI was 383.93±63.82, 333.02±71.79, 312.48±64.13, 312.79±55.49, and 304.71±74.63 on the left cheek and 365.4±60.42, 337.6±69.4, 318.75±57.92, 319.84±40.48, and 308.82±83.46 at baseline and 1, 2, 4, and 8 weeks after treatment, respectively. The EI score decreased from 1 week after treatment and continued to decrease until 8 weeks after treatment (p<0.05) (Fig. 2).
The score of erythema severity (ES) was 1.9±0.27, 1.5±0.51, 1.26±0.47, 1.26±0.39, 1.23±0.48 at baseline and 1, 2, 4, and 8 weeks after the procedure, respectively. The score decreased from 1 week after treatment, and the effect was maintained at 8 weeks, with the maximal effect at 4 weeks after treatment. The ES measured at 1, 2, 4, and 8 weeks decreased significantly for all time points versus baseline (p<0.05) (Fig. 3). The score of telangiectasia severity (TS) started to decrease from 1 week after treatment and decreased steadily up to 8 weeks, with results of 1.65±0.52, 1.25±0.48, 0.90±0.38, 0.90±0.28, and 0.94±0.44 at baseline, 1, 2, 4, and 8 weeks after the procedure, respectively. The decrement of TS in the course of the study was statistically significant (p<0.05) (Fig. 4).
In the satisfaction evaluation, subjects rated the results as 2.45±0.54 at week 1, 2.79±0.71 at week 2, 2.84±0.55 at week 4, and 2.94±0.56 at week 8, which showed a steady increase in satisfaction between slightly satisfied (2) and moderately satisfied (3).
During the study period, three patients complained of unnatural facial expressions and discontinued participation. Their mild facial paralysis improved within three months without further treatment. Of the total subjects including those who completed the clinical trial early, there was no serious complication, and physical examination by a dermatologist did not reveal any skin abnormalities such as erythema, rash, or itching.
The facial redness and flushing of ETR are difficult to treat with a single medical therapy; therefore, other treatment modalities may be especially important for successfully controlling this condition. In this study, we evaluated the efficacy and safety of BTX injection for treating the recalcitrant facial redness of ETR. We planned this clinical study based on our experience of successfully treating ETR patients who did not respond to other treatments with BTX10. In the present study, the severity of erythema and telangiectasia, evaluated by a non-treating physician, began to decrease one week after treatment, showed the maximum effect 4 weeks after treatment, and continued to show an effect up to 8 weeks after treatment. EI measured by mexameter started to decrease 1 week after treatment and decreased continuously up to 8 weeks after treatment. Patients' satisfaction scores also increased in proportion to objective indexes from 1 week after treatment to 8 weeks after treatment.
BTX's molecular mode of action mainly includes extracellular binding to glycoprotein structures on cholinergic nerve terminals and intracellular blockade of ACh secretion. ACh affects vasodilation by several mechanisms, including activation of endothelial nitric oxide synthase and prostaglandin production11, and exogenous ACh increases blood flow in human skin. The mechanism for increased skin blood flow is co-transmission by sympathetic cholinergic nerves and the release of acetylcholine and one or more cotransmitters. Vasoactive intestinal peptide (VIP) and PACAP, as cotransmitters for the active vasodilator system, are found in skin and can be co-localized with acetylcholine12.
In rosacea, activation of precapillary arterioles results in vasodilation (flushing, erythema), and various pathways involved in vasodilation have been found to be significantly upregulated including modulation by neuropeptides, tryptophan metabolites, lipids, or radical oxygen species1314. Drummond and Su15 suggested that activation of nociceptive nerve fibers contributes to skin sensitivity, and that axon reflexes augment flushing in patients with rosacea. BTX can reduce axon reflexes and neurogenic vasodilation in human skin1617.
The critical mediators that induce sustained flushing in rosacea patients may be potent vasoregulatory neuropeptides such as PACAP, VIP, or CGRP, as well as SP that may also contribute to the edema1. PACAP, one of the most potent vasodilators, which is increased up to greater than 20-fold in all subtypes of rosacea, is released by activated endothelial cells and degranulated mast cells18.
Gene analysis of classic neuromediators and receptors revealed that several neuropeptides, vasoactive amines, or transient receptor potentials (TRPs) involved in vasoregulation and pain are expressed by resident skin cells and immune cells in rosacea18. TRPV1 is involved in synaptic transmission, in which it modulates neurotransmitter release, plasticity, and vesicle recycling19. Recent studies have shown that dermal immunolabeling of TRPV2 and TRPV3 and gene expression of TRPV1 were significantly increased in ETR, and dysregulation of TRPV channels might be critically involved in the initiation and/or development of rosacea. TRPV1 can be activated by heat, ethanol, or spicy food, and activation of TRPV1 caused release of SP and CGRP, important mediators of neurogenic inflammation and pain132021.
Recently, researchers have found that TRPV1 activation increased CGRP release, and that BTX was able to block this process2223. An animal study revealed that subcutaneous BTX injection decreased TRPV1-immunoreactive neurons in rat trigeminal ganglions24. BTX can also inhibit CGRP release, which leads to pain alleviation through the structural impairment and dysfunction of SNAP-2522.
In our study, no significant adverse events except mild pain and bruising were observed during the course of the study, but undesired paralysis of facial muscles was reported in three patients. The degree of facial paralysis was such that these patients felt unnatural when they made a certain expression (e.g., smiling), and the condition resolved without any special treatment. As judged by the evaluating physician, the degree of facial paralysis was mild; however, the three patients stopped participating in the study because of their discomfort. Meanwhile, Dayan et al.2 reported that intradermal injection of 8~12 units of BTX on each cheek could provide clinical improvement of rosacea. In another report, 15 units of BTX were injected intradermally into each cheek of facial flushing patients to confirm good therapeutic efficacy and safety25. Our study was conducted in Koreans, and the frequency of patients complaining of paralysis of facial muscles was high, even though 10 units, which was a similar or lower dose than previous reports, was used in each cheek. Considering the effects of racial differences, further studies on the treatment volume and depth are needed. Particularly interesting in this study was that all three patients who complained of discomfort due to facial paralysis and stopped participation were young women in their 20s or early 30s. Therefore, when considering BTX treatment for ETR of young Asian women, more thorough and careful counseling and explanation should be conducted. After the treatment is decided, a method of retouching two weeks after administering a smaller amount of BTX is recommended.
This research have several limitations in the general application of the study findings. Firstly, the number of patients was too small. Secondly, This pilot study was neither randomized nor placebo-controlled. To overcome the limitation, larger number of patients and well-designed study are necessary.
In conclusion, no single optimal treatment has been indicated for ETR; therefore, intradermal BTX injection is expected to be a reasonable addition to the therapeutic options for treating these conditions, especially when other established therapies have failed. Additional large-scale studies will be needed in the future to obtain information on the therapeutic effect for several types of rosacea, duration of effect, retouch interval, and optimal dose of BTX injections.
Figures and Tables
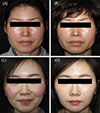 | Fig. 1Two representative cases of clinical photographs. (A) and (C) before the treatment. (B) and (D) clinical improvement of rosacea after 8 weeks of botulinum toxin injection. |
 | Fig. 2Comparison of mean difference of repeated measure variables of baseline Erythema Index (EI), 1 week EI, 2 week EI, 4 week EI, and 8 week EI expressed as mean±standard deviation in right (R) cheek (A) and left (L) cheek (B). |
References
1. Schwab VD, Sulk M, Seeliger S, Nowak P, Aubert J, Mess C, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011; 15:53–62.

2. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012; 11:e76–e79.
3. Bloom BS, Payongayong L, Mourin A, Goldberg DJ. Impact of intradermal abobotulinumtoxinA on facial erythema of rosacea. Dermatol Surg. 2015; 41:Suppl 1. S9–S16.

4. Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008; 7:847–850.
5. Oh YJ, Lee NY, Suh DH, Koh JS, Lee SJ, Shin MK. A split-face study using botulinum toxin type B to decrease facial erythema index. J Cosmet Laser Ther. 2011; 13:243–248.

6. Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon. 2000; 38:245–258.

7. Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache. 2004; 44:35–42.

8. Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005; 174:977–982.
9. Gessert CE, Bamford JT. Measuring the severity of rosacea: a review. Int J Dermatol. 2003; 42:444–448.

10. Park KY, Hyun MY, Jeong SY, Kim BJ, Kim MN, Hong CK. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015; 230:299–301.

11. Kellogg DL Jr, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, et al. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995; 77:1222–1228.

12. Kellogg DL Jr, Zhao JL, Wu Y, Johnson JM. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J Appl Physiol (1985). 2012; 113:1512–1518.

13. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013; 69:6 Suppl 1. S15–S26.

14. Steinhoff M, Buddenkotte J, Aubert J, Sulk M, Novak P, Schwab VD, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011; 15:2–11.

15. Drummond PD, Su D. Endothelial and axon reflex vasodilatation to acetylcholine in rosacea-affected skin. Arch Dermatol Res. 2012; 304:133–137.

16. Krämer HH, Angerer C, Erbguth F, Schmelz M, Birklein F. Botulinum toxin A reduces neurogenic flare but has almost no effect on pain and hyperalgesia in human skin. J Neurol. 2003; 250:188–193.

17. Tugnoli V, Capone JG, Eleopra R, Quatrale R, Sensi M, Gastaldo E, et al. Botulinum toxin type A reduces capsaicinevoked pain and neurogenic vasodilatation in human skin. Pain. 2007; 130:76–83.

18. Seeliger S, Buddenkotte J, Schmidt-Choudhury A, Rosignoli C, Shpacovitch V, von Arnim U, et al. Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo. Am J Pathol. 2010; 177:2563–2575.
19. Goswami C, Rademacher N, Smalla KH, Kalscheuer V, Ropers HH, Gundelfinger ED, et al. TRPV1 acts as a synaptic protein and regulates vesicle recycling. J Cell Sci. 2010; 123:2045–2057.

20. Sulk M, Seeliger S, Aubert J, Schwab VD, Cevikbas F, Rivier M, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012; 132:1253–1262.

21. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004; 173:2909–2912.

22. Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, et al. Activation of TRPV1 mediates calcitonin generelated peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009; 29:4981–4992.

23. Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, et al. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis. 2012; 48:367–378.

24. Li X, Coffield JA. Structural and functional interactions between transient receptor potential vanilloid subfamily 1 and botulinum neurotoxin serotype A. PLos One. 2016; 11:e0143024.

25. Eshghi G, Khezrian L, Alirezaei P. Botulinum toxin A in treatment of facial flushing. Acta Med Iran. 2016; 54:454–457.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download