Abstract
Background
Rosacea is associated with chronic systemic disease. However, research is lacking in Asian countries.
Objective
To evaluate the association between rosacea and cardiovascular diseases (CVDs) related systemic comorbidities, and the use of antihypertensive and antihyperlipidemic drugs in Korea.
Methods
A five-year retrospective study, using hospital database, was conducted in five medical centers for five years. Totally 1,399,528 patients were evaluated.
Results
The overall frequency for diagnosed rosacea was 0.18% over five years (2,536 rosacea patients). Patients with diabetes and patients with dyslipidemia were more likely to have rosacea (odd ratio [OR] 2.724, 95% confidence interval [CI] 1.295~5.730, p=0.016; OR 1.788, 95% CI 1.445~2.212, p<0.001). Patients with CVD were less likely to have rosacea (OR 0.431, 95% CI 0.244~0.760, p=0.003). Patients with α-blocker prescriptions and patients with β-blocker prescriptions showed a tendency diagnosed with rosacea frequently (OR 1.963, 95% CI 1.200~3.212, p=0.006; OR 3.939, 95% CI 3.512~4.419, p<0.001). Patients with [beta]-hydroxy-[beta]-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, and those with fibrate, were prone to have rosacea (OR 1.599, 95% CI 1.390~1.839, p<0.001; OR 1.660, 95% CI 1.056~2.609, p=0.026). As adjusted results, among the patients who took HMG-CoA reductase inhibitor without dyslipidemia, rosacea was less likely to be diagnosed (OR 0.780, 95% CI 0.620~0.982, p=0.034).
Rosacea is a chronic inflammatory skin disease and symptoms present in variable degrees of facial erythema, telangiectasias, papules, and pustules123. Although the exact pathophysiology of rosacea has not been established yet, vascular reactivity, sun exposure, inflammations, micro-organisms such as Helicobacter pylori, Demodex folliculorum, Staphylococcus epidermidis and Bacillus oleronius, and neuro-psychogenic factor seem to be involved12345. Besides a variety of other suggested mechanisms about pathogenesis, evidence points to a key role in rosacea of neurovascular dysregulation and neurogenic inflammation in the vasodilative pathomechanism4. The proposed pathogenic factors have not yet been proven. Recently, associations between various chronic inflammatory diseases, such as rheumatoid arthritis (RA) and psoriasis, with cardiovascular disease (CVD) were reported567. Systemic studies to show the associations of rosacea with extracutaneous diseases are few. Rosacea can appear in all ethnic groups but previous studies reported that rosacea is less common among Asians or Mongoloids. It might imply a different pathophysiology related to genetic background or genetic susceptibility. Because most previous studies tried to reveal associations between CVD and rosacea among Caucasians, it is needed and meaningful to determine the relationship between CVD, its drugs, and rosacea among Asians8.
Therefore, we tried to investigate a group of rosacea patients among the patients who had risk factors of CVD and who were prescribed drugs for chronic systemic disease in South Korea.
We conducted a five-year retrospective, multi-institutional pooled analysis in five hospitals. A total of 1,399,528 patients in Hallym, Doangtan, Hangang, Chuncheon, and Kangnam Sacred Heart Hospitals between January 2011 and December 2015 were included. Among them, we selected rosacea patients from the hospital database, a program named Clinical Data Warehouse (CDW) with the International Classification of Diseases (ICD) diagnostic codes for “rosacea” (L711, L718 or L719). The extracted rosacea population consisted of 2,536 patients who firstly recorded the ICD codes for rosacea as new patient on dates between study periods.
We obtained patients with cardiovascular risk factors from the CDW database. We used ICD codes, including I101~I109 hypertension, E780~E785, E788 dyslipidemia or hyperlipidemia, I250~I259 atherosclerotic heart disease (chronic ischemic heart disease), I1630~I639 cerebral infarction, E10~E14 diabetes, and I740~I749 arterial embolism and thrombosis (peripheral arterial occlusive disease). We collected patients' data including demographic data (age at diagnosis, gender) and medical history (comorbidities which are the above-mentioned diseases extracted as ICD code, disease type and duration). After that, among them, patients diagnosed with rosacea were extracted again. The time at which diagnosed with rosacea was set up as an index date. Only those patients diagnosed with known systemic or cardiovascular disease before the index date were included.
Drug prescriptions of patients are generated electronically via computer, ensuring a virtually complete drug history. All the target prescribed drugs were investigated by subgroups such as antihypertensive drugs and antihyperlipidemic drugs. Table 1 shows which drugs we used to extract data. We extracted rosacea patients again just as above. Only patients prescribed certain drugs before the index date were included.
To consider the interactive effect of disease and drugs, subdivided analysis was done. Each disease and a drug were paired (e.g., a hypertension and antihypertensive drug, hypertension and aspirin, CVD and aspirin, dyslipidemia and [beta]-hydroxy-[beta]-methylglutaryl coenzyme A [HMG-CoA] reductase inhibitor), with concern that each other acts as a confounding factor.
To establish the frequency of the diagnosed rosacea in the population, we assessed the number of rosacea patients during five years by year and by sex.
The protocol was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital (IRB no. 2016-06-77).
We estimated the frequency of diagnosed rosacea using the Poisson regression analysis. We use the genlin command to estimate a Poisson regression model. We have one continuous predictor and one categorical predictor. The factor, categorical variable was sex and cell covariate, continuous variable was year. The “cases of diagnosed rosacea” is the dependent variable, whereas “sex” is nominal independent variable. The probability distribution is "Poisson" and the link function is the natural logarithm. The logged variable, “Ln_ total (total number of patients)”, is used as the offset variable. Using the chi-square test, the proportion of rosacea-exhibiting and rosacea-free patients for each disease and each drug was calculated. The significance level set for all analyses was p<0.05. All statistical analyses were conducted using PASW Statistics 18 (IBM Co., Armonk, NY, USA).
The study population encompassed 2,536 patients with rosacea, of whom 1,745 patients (68.81%) were female. A total of 1,399,528 patients visited hospitals during the five target years. The vast majority (2,151 or 84.81%) of patients with rosacea were above the age of 30 years at the index date. Their average age was 47.21±15.01 years.
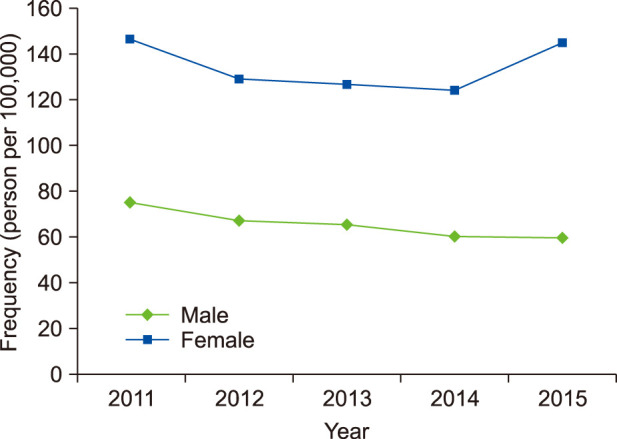
The frequency of diagnosed rosacea in the study population was 0.18% (181.20 per 100,000) during the five years from 2011 to 2015. Fig. 1 shows changes in the frequency of diagnosed rosacea. It was higher in women (0.25%, 1,745/705,748) than in men (0.11%, 791/693,780). In women, it was 2.062 times significantly higher than in men (p<0.001). The frequency of diagnosed rosacea in each year did not differ significantly over the five years (p=0.132).
Of the 1,399,528 patients, some were selected depending on their comorbidities and their prescribed drugs. Table 2 presents rosacea patients who were extracted in relation to chronic systemic diseases and prescribed drugs.
Patients with diabetes (odd ratio [OR] 2.724, 95% confidence interval [CI] 1.295~5.730, p=0.016), and patients with dyslipidemia (OR 1.788, 95% CI 1.445~2.212, p<0.001), were significantly more likely to have rosacea. Patients with hypertension was more likely to have rosacea, but this was not statistically significant (OR 1.205, 95% CI 0.987~1.470, p=0.073). Meanwhile, patients with cerebral infarction were statistically less prone to have rosacea (OR 0.431, 95% CI 0.244~0.760, p=0.003) (Fig. 2).
Patients with one or more recorded α-blockers, and patients with β-blocker (BB) prescriptions, showed a significantly greater tendency to be diagnosed with rosacea compared with patients without such prescriptions (OR 1.963, 95% CI 1.200~3.212, p=0.006; OR 3.939, 95% CI 3.512~4.419, p<0.001). Moreover, patients with HMG-CoA reductase inhibitor and patients with fibrate were significantly more prone to have rosacea (OR 1.599, 95% CI 1.390~1.839, p<0.001; OR 1.660, 95% CI 1.056~2.609, p=0.026). Patients with calcium channel blocker (CCB, OR 1.061, 95% CI 0.975~1.629, p=0.611) and patients with diuretics (OR 1.066, 95% CI 0.825~1.377, p=0.596) were slightly more likely to have rosacea, but these were not statistically significant (Fig. 3).
The proportions of rosacea and rosacea-free patients for each antihypertensive or antihyperlipidemic drug are summarized in Table 3. Among the patients with one or more recorded BB β1 and β2 (OR 5.106, 95% CI 4.380~5.953, p<0.001), non-selective BB and α1-blocker (OR 6.288, 95% CI 5.245~7.539, p<0.001), and V2 arginine vasopressin receptor antagonists (OR 11.430, 95% CI 1.584~83.201, p=0.002), rosacea was significantly more likely to be diagnosed (Fig. 4). No drugs showed a significant tendency for users less diagnosed with rosacea, compared with patients not taking them. When divided by sex, patients taking carbonic anhydrase inhibitors showed significantly increased tendency to develop rosacea only among male patients. In contrast, among patients taking V2 arginine vasopressin receptor antagonists, only female patients exhibited increased tendency to develop rosacea.
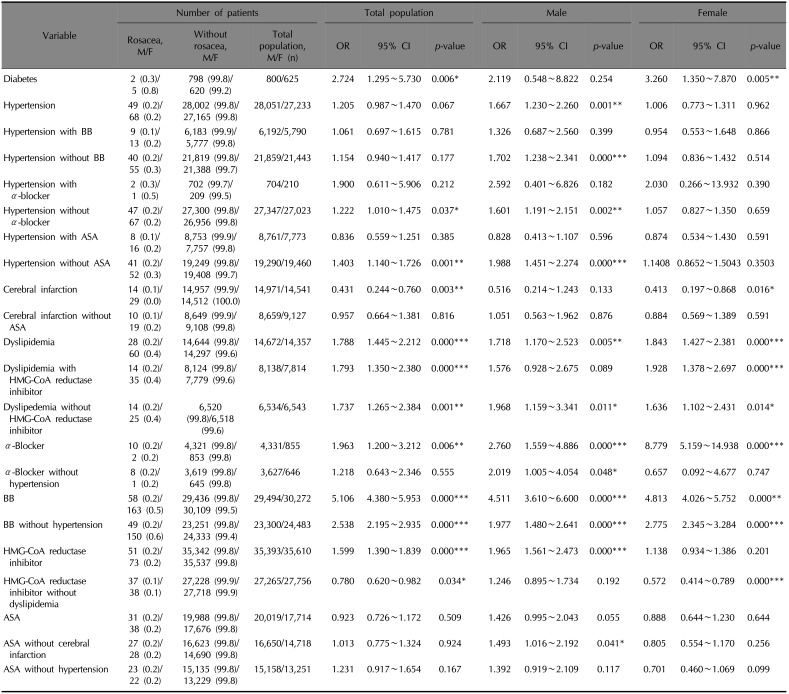
We divided into male and female, and find each increased odds ratio for rosacea development in patients with dyslipidemia (OR 1.718, 95% CI 1.170~2.523, p=0.005 for males and OR 1.843, 95% CI 1.427~2.381, p<0.001 for females). Also of interest, only female patients with diabetes mellitus (DM) (OR 3.260, 95% CI 1.350~7.870, p=0.005) and cerebral infarction (OR 0.413, 95% CI 0.197~0.868, p=0.016) showed increased and decreased tendency to develop rosacea. In the case of hypertension, only males showed an increased tendency to develop rosacea (OR 1.667, 95% CI 1.230~2.260, p=0.001).
When divided by sex, male patients with every prescribed drug mentioned above (except diuretics) showed a significantly increased tendency to develop rosacea. For female patients, only those with α-blockers (OR 8.779, 95% CI 5.159~14.938, p<0.001) and BB (OR 4.813, 95% CI 4.026~5.752, p<0.001) prescriptions were more likely to have rosacea.
Even when paired divided analyses were carried out with confounding factors, hypertension, dyslipidemia, BB showed significantly increased the tendency to develop rosacea. On the other hand, among the patients who took HMG-CoA reductase inhibitor without dyslipidemia, rosacea was less likely to be diagnosed (OR 0.780, 95% CI 0.620~0.982, p=0.034) (Fig. 5, Table 4).
Reported incidence and prevalence of rosacea vary between countries, ranging from 0.06% to 22%39. Rosacea affects up to 15% of the general populationof fair-skinned northern European heritage2. The overall incidence rate for diagnosed rosacea in the UK was 1.65/1,000 person-years. The prevalence of rosacea in the general population of Germany and Russia was 12.3% and 5.0%, respectively10. Rosacea seems relatively common among Caucasians and occurs less frequently in other ethnicities. In South Korea, Koo et al.11 (1997) studied 7,797 patients who visited a dermatology clinic for five years. Among these, 133 patients were diagnosed with rosacea; so the frequency of rosacea was 1.7%. In another study done by Kim et al.12, 8,491 patients who visited the department of dermatology were studied for two years, and 82 patients were diagnosed with rosacea, revealing the frequency of rosacea to be 0.97%. In our study, the frequency of rosacea over five years in our group of hospitals was 0.18%. The relatively low incidence may be due to a patient missing from the tertiary hospital. The study data included only patients who visited the five hospitals. There might be patients who did not come to dermatologists due to mild symptoms and signs13. Also, Korean people, most of whom are Asian, may have different prevalence rates11,12.
Our study confirmed a significant association of rosacea and dyslipidemia. There are some explanations about the association between hyperlipidemia and rosacea, such as high rate of alcohol consumption and genetic factors; among other, unknown factors1. Systemic inflammation can lead to structural changes in lipoproteins, negatively affecting their ability to remove cholesterol14. In a case-control study of Duman et al.1, high total cholesterol (>200 mg/dl) and low-density lipoprotein cholesterol (>130 mg/dl) were significantly more common in the rosacea patients compared to controls. Rainer et al.2 also found a significant association between hyperlipidemia and rosacea in a skin severity-dependent manner in a case-control study (OR 6.8, 95% CI 1.900~24.600, p=0.003). For these reasons, chronic inflammation from rosacea could be the cause of frequent dyslipidemia.
Antihyperlipidemic drugs, HMG-CoA reductase inhibitors and fibrates, showed significant association with increased diagnosis of rosacea. However, patients with dyslipidemia were significantly more likely to have rosacea; so interpretation should be careful. Interestingly, after subdivision, patients who were prescribed HMG-CoA reductase inhibitor but have no dyslipidemia showed less likely to have rosacea compared with those who were not prescribed HMG-CoA reductase inhibitor or have dyslipidemia. When divided into male and female, this result was not observed in male, but in female. This difference may be due to the distinct skin physiology in men and women15, and physiologic differences between male and female rosacea16. Regarding the HMG-CoA reductase inhibitor, statin; it is reported to be effective against many other dermatologic diseases including psoriasis, eczema, graft-versus-host disease, uremic pruritus, vitiligo, and hirsutism17. Also, its topical forms are employed in the treatment of acne, seborrhea, rosacea, and rhinophyma17. With immunomodulatory effects18, its systemic anti-inflammatory action in inflammatory arthritis18 and allergic asthma19 has been demonstrated17. Additionally, statin inhibits the activity of oxidant enzymes20, and up-regulates the activity of antioxidant enzymes17. Statin also has a biphasic effect on angiogenesis in human epidermal microvessel endothelial cells18; low concentrations tend to increase angiogenesis but higher doses induce inhibition of vascular endothelial growth factor synthesis21. Also, statins inhibit the expression and secretion of MMP-1,-2,-3 and -9 from vascular smooth muscle cells and macrophages22, compared with fibrate (no inhibition)23. MMPs play a major role in remodeling the extracellular matrix2425 and also in the etiopathogenesis of rosacea; so the protective effects of statin on developing rosacea in patients with hyperlipidemia should be considered. Because our results with or without dyslipidemia showed different results in the effect on incidence of rosacea, controlled experimental data will be needed.
Our study revealed that patients with hypertension were not likely to have rosacea. In Duman's case-control study, regarding hypertension there was no significant difference between the rosacea group and control group1. Otherwise, Rainer et al.2 revealed patients with rosacea were more likely to have hypertension with severity-dependent manner. Underlying inflammatory cytokines and metabolic, immune, and endocrine changes might provide explanations. In our study, patientstaking α-blockers and patients with BB prescriptions showed a significantly greater tendency to be diagnosed with rosacea. Patients with CCB, diuretics, angiotensin-converting-enzyme inhibitor (ACEi), and angiotensin II receptor blocker (ARB) did not show any significant tendency regarding rosacea. BBs are recommended as an off-label treatment for erythematoutelangiectiatic rosacea26. In some case-control research, ORs for incident rosacea slightly decreased among patients with BB27. Use of CCBs, by the way, is commonly discouraged in patients with rosacea because they are considered a trigger or exacerbating factor262829. Associations between the risk of incident rosacea and such antihypertensive drugs have been reported recently27; however, the evidence is limited and still controversial2729.
Our study shows patients with DM have a significantly higher probability of experiencing rosacea. However, Duman et al.1 showed there were no significant differences between rosacea group and control group in fasting blood glucose levels and DM. Meanwhile, Spoendlin et al.30 found rosacea cases and controls had recorded DM diagnoses revealing decreased ORs. This further decreased with increasing hemoglobin A1c values and disease duration. The underlying mechanism seems related to the degree of endothelial dysfunction and thus impaired vasodilation. Rosacea has a vasodilatory component whereas DM is associated with impaired vasodilation congruent with the degree of endothelial dysfunction31. These suggest a decreased rosacea risk in DM patients but some residual confounding or chance cannot entirely be ruled out.
The incidence rate of rosacea among ischemic stroke and hemorrhagic stroke patientswas reported to be 1.19 and 1.11, respectively, but both were without statistical significance32. In our study, patients with cerebral infarction were statistically less prone to have rosacea. Meanwhile, aspirin slightly raised the OR in the male population without CVD. CVD is also associated with other risk factors or confounding factors, such as alcohol, smoking, and obesity, which are difficult to interpret. Not many studies on these have been reported and they are still controversial. As suggested mechanism, aspirin blocks synthesis of prostaglandin, which induces vasodilation33,34.
The limitations of this study are as follows. Firstly, this study included a population of patients who visited hospitals, not the general population. However, other cohort or case-control studies using national databases also included populations of patients who visited hospitals. Second, the records of ICD codes might be incomplete (e.g., many physicians might have forgotten to enter ICD codes and it might be possible that the diagnosis of specific disease has been included in the diagnosis for the insurance even without that disease. Because of those reasons, there may be missed cases. Also, here are limitations in that code may be entered for insurance claims. Therefore, it was not possible to exclude other inflammatory diseases. Additional case-control studies are needed. Also, in some cases, there may be use of preventive medicines used differently from the proposals of the National Health Insurance). Third, using the chi-square test, we could confirm the relations between each group due to limitations in the use of medical records. Later on, case-control study or cohort study with multiple logistic regression analysis would be needed to check the odd ratio or relative rate. Also, we could not check the exact date of occurrence of the disease or distinguish new patients from patients with existing disease. So, it is unclear whether rosacea would be the prodrome sign or one of the clinical feature/sequel of specific chronic systemic diseases, and rosacea can be regarded as one of drug side reaction. At last, because this study is limited to patients seen in a hospital setting, patients with more severe rosacea might be included. Also, in this study, we did not survey the general population and only studied patients who visited five hospitals, which are located locally in some urban areas. The selection of hospitals is not the probability allocation, so the data of five hospitals can not represent those of South Korea. Also, these data only showed not the prevalence of rosacea but the frequency of diagnosed rosacea. Due to these limitations, an interpretation will need to be careful. It might be thought to have only the significance as a preliminary experiment. In addition, it was hard to evaluate patients taking a combination of drugs in one pill, so we could not include antihypertensive combinations such as ACEi with CCB agents, ARB with CCB, and so on. Additional study with the number of prescriptions or dose amounts will also be needed. Also, classification of rosacea into subtypes, using clinical data, such as clinical picture or histopathology results, and consideration for the influencing factors, such as environment, temperature, patient's age, occupation and habits, cosmetics and topical agent might be needed in future studies.
This study determined the correlation of chronic systemic diseaseand rosacea in South Korean patients. We also demonstrated associations between rosacea and drugs for risk factors of CVDs. Patients with hyperlipidemia, in particular, were more likely to have rosacea; and patients prescribed with HMG-CoA reductase inhibitor seemed less likely to have rosacea. These results provide good evidence for explanations about the potential of rosacea occurrence when prescribing medications to patients with chronic systemic disease.
ACKNOWLEDGMENT
This study was supported by grants of the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1A2B4006252, 2018R1C1B6007998), Korea Healthcare technology R&D project, funded by Ministry of Health & Welfare, Republic of Korea (HI17C0597), and the Hallym University Research Fund (HURF-2017-35, HURF-2017-52, HURF-2017-83).
References
1. Duman N, Ersoy Evans S, Atakan N. Rosacea and cardiovascular risk factors: a case control study. J Eur Acad Dermatol Venereol. 2014; 28:1165–1169. PMID: 23909954.

2. Rainer BM, Fischer AH, Luz Felipe da Silva D, Kang S, Chien AL. Rosacea is associated with chronic systemic diseases in a skin severity–dependent manner: results of a case-control study. J Am Acad Dermatol. 2015; 73:604–608. PMID: 26256428.
3. Rueda LJ, Motta A, Pabón JG, Barona MI, Meléndez E, Orozco B, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017; 56:510–513. PMID: 28239916.

4. Schwab VD, Sulk M, Seeliger S, Nowak P, Aubert J, Mess C, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011; 15:53–62.

5. Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2009; 31:1000–1006. PMID: 20037179.

6. Ozbalkan Z, Efe C, Cesur M, Ertek S, Nasiroglu N, Berneis K, et al. An update on the relationships between rheumatoid arthritis and atherosclerosis. Atherosclerosis. 2010; 212:377–382. PMID: 20430394.

7. Itani S, Arabi A, Harb D, Hamzeh D, Kibbi AG. High prevalence of metabolic syndrome in patients with psoriasis in Lebanon: a prospective study. Int J Dermatol. 2016; 55:390–395. PMID: 26748974.

8. Merticariu A, Marinescu L, Giurcăneanu C. Rosacea and its comorbidities. J Transl Med Res. 2016; 21:17–23.

9. Spoendlin J, Voegel JJ, Jick SS, Meier CR. Diabetes mellitus, antidiabetic drugs and the risk of developing rosacea. Pharmacoepidemiol Drug Saf. 2012; 21:275–276.
10. Tan J, Schöfer H, Araviiskaia E, Audibert F, Kerrouche N, Berg M. Prevalence of rosacea in the general population of Germany and Russia - the RISE study. J Eur Acad Dermatol Venereol. 2016; 30:428–434. PMID: 26915718.
11. Koo BS, Kwon HJ, Kim BC, Lee KS, Song JY. A clinical study of 133 patients with rosacea. Korean J Dermatol. 1997; 35:405–410.
12. Kim TH, Hwang SM, Lee WS, Ahn SK, Choi EH. A clinical study of rosacea. Korean J Dermatol. 2000; 38:583–588.
13. Kim MS, Kim BS, Koh WS, Lee SS, Seo SL, Chun DK, et al. Rosacea: clinical study of 67 cases. Ann Dermatol. 2001; 13:39–43.
14. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002; 105:1135–1143. PMID: 11877368.

15. Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women: in vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int J Cosmet Sci. 2013; 35:477–483. PMID: 23713991.
16. BORRIE P. Rosacea with special reference to its ocular manifestations. Br J Dermatol. 1953; 65:458–463. PMID: 13115586.

17. Jowkar F, Namazi MR. Statins in dermatology. Int J Dermatol. 2010; 49:1235–1243. PMID: 20964647.

18. Namazi MR. Statins: novel additions to the dermatologic arsenal? Exp Dermatol. 2004; 13:337–339. PMID: 15186318.

19. McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004; 172:2903–2908. PMID: 14978092.

20. Stoll LL, McCormick ML, Denning GM, Weintraub NL. Antioxidant effects of statins. Drugs Today (Barc). 2004; 40:975–990. PMID: 15645009.

21. Katsumoto M, Shingu T, Kuwashima R, Nakata A, Nomura S, Chayama K. Biphasic effect of HMG-CoA reductase inhibitor, pitavastatin, on vascular endothelial cells and angiogenesis. Circ J. 2005; 69:1547–1555. PMID: 16308507.

22. Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1,-2,-3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003; 23:769–775. PMID: 12663370.
23. Koh KK, Ahn JY, Jin DK, Han SH, Kim HS, Choi IS, et al. Comparative effects of statin and fibrate on nitric oxide bioactivity and matrix metalloproteinase in hyperlipidemia. Int J Cardiol. 2004; 97:239–244. PMID: 15458690.

24. Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995; 77:863–868. PMID: 7554139.

25. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002; 90:251–262. PMID: 11861412.
26. Odom R, Dahl M, Dover J, Draelos Z, Drake L, Macsai M, et al. Standard management options for rosacea, part 1: overview and broad spectrum of care. Cutis. 2009; 84:43–47. PMID: 19743724.
27. Spoendlin J, Voegel J, Jick S, Meier CR. Antihypertensive drugs and the risk of incident rosacea. Br J Dermatol. 2014; 171:130–136. PMID: 24428524.

28. Powell FC. Clinical practice. Rosacea. N Engl J Med. 2005; 352:793–803. PMID: 15728812.
29. Natale F, Cirillo C, Granato C, Concilio C, Siciliano A, Credendino M, et al. Worsening of rosacea in patients treated with dihydropyridine calcium channel blockers: a clinical observation. Hypertens Res. 2011; 34:790–791. PMID: 21451527.

30. Spoendlin J, Voegel JJ, Jick SS, Meier CR. Risk of rosacea in patients with diabetes using insulin or oral antidiabetic drugs. J Invest Dermatol. 2013; 133:2790–2793. PMID: 23657502.

31. Browne D, Meeking D, Shaw K, Cummings M. Review: endothelial dysfunction and pre-symptomatic atherosclerosis in type 1 diabetes — pathogenesis and identification. Br J Diabetes Vasc Dis. 2003; 3:27–34.

32. Guarrera M, Parodi A, Cipriani C, Divano C, Rebora A. Flushing in rosacea: a possible mechanism. Arch Dermatol Res. 1982; 272:311–316. PMID: 6219630.

33. Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Assessment of the risk of cardiovascular disease in patients with rosacea. J Am Acad Dermatol. 2016; 75:336–339. PMID: 27444070.

34. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003; 110:255–258. PMID: 14592543.

Fig. 2
Risk of diagnosed rosacea according to chronic systemic diseases. IHD: ischemic heart disease, CVD: cardiovascular disease. *p<0.05.
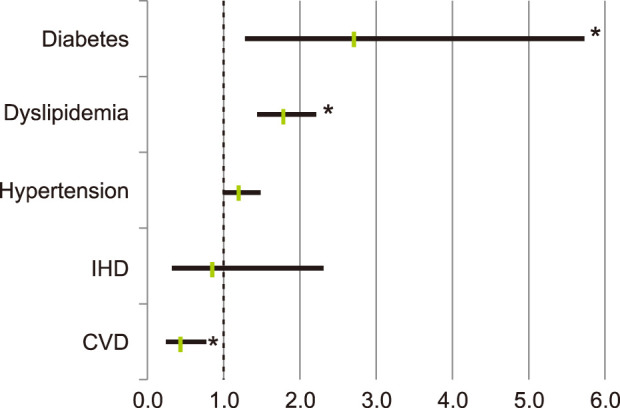
Fig. 3
Risk of diagnosed rosacea in relation to medications such as antihypertensive and antihyperlipidemic drugs. CCB: calcium channel blocker, ACEi: angiotensin-converting-enzyme inhibitor, ARB: angiotensin II receptor blocker, HMG-CoA: [beta]-hydroxy-[beta]-methylglutaryl coenzyme A, ASA: aspirin. *p<0.05.
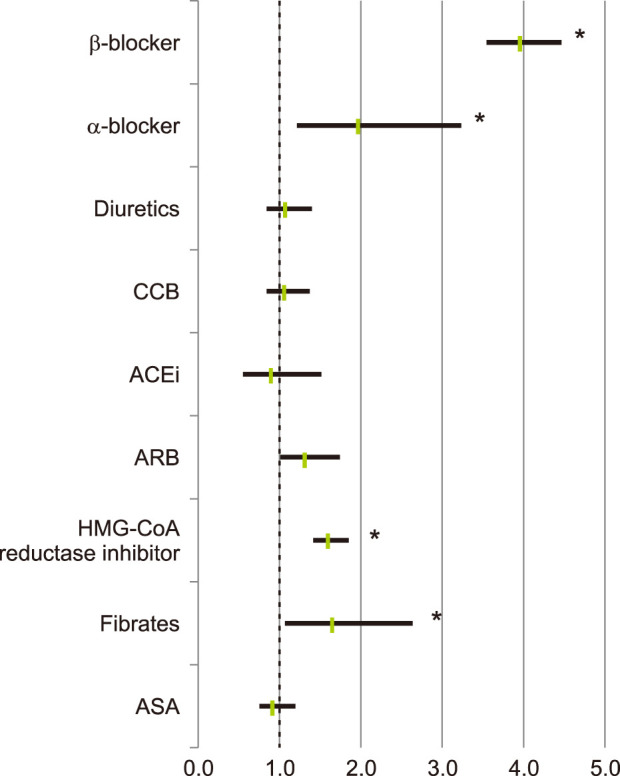
Fig. 4
(A) Risk of diagnosed rosacea according to specific diuretics, (B) risk of diagnosed rosacea according to specific α-blockers and β-blocker, classified drugs by subtypes. *p<0.05.
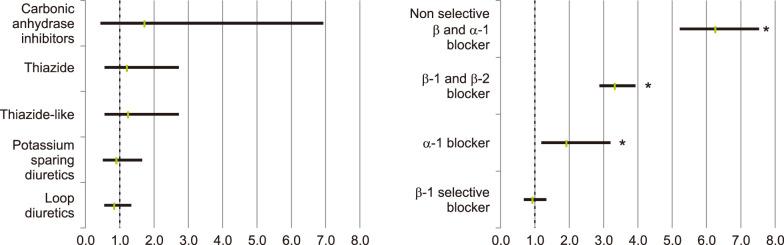
Fig. 5
Odds ratio for the risk of diagnosed rosacea in (A) total population, (B) males, and (C) females, after subdivided analysis. HMG-CoA: [beta]-hydroxy-[beta]-methylglutaryl coenzyme A, CVA: cerebrovascular attack, ASA: aspirin. *p<0.05.
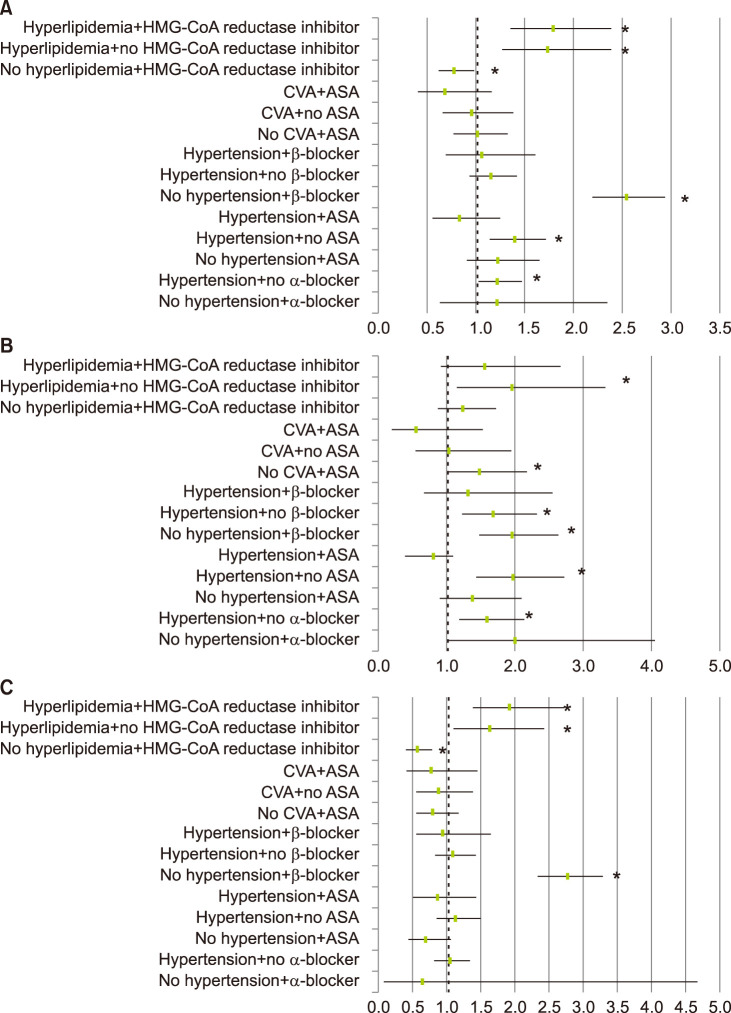
Table 1
Classification of drugs
Table 2
The risk of diagnosed rosacea according to chronic systemic diseases, which is related to cardiovascular risk factors, and drugs
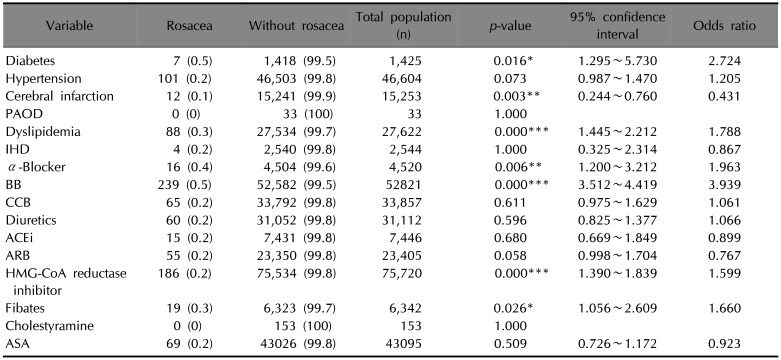
Values are presented as number (%). PAOD: peripheral arterial occlusive disease, IHD: ischemic heart disease, BB: β-blocker, CCB: calcium channel blocker, ACEi: angiotensin-converting-enzyme inhibitor, ARB: angiotensin II receptor blocker, HMG-CoA: [beta]-hydroxy-[beta]-methylglutaryl coenzyme A, ASA: aspirin. *p<0.05, **p<0.01, ***p<0.001.
Table 3
The risk of diagnosed rosacea according to classified drugs by subtypes
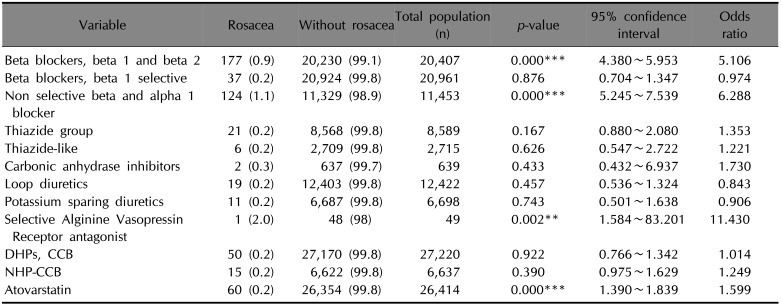
Table 4
The risk of diagnosed rosacea according to chronic systemic diseases, which is related to cardiovascular risk factors and drugs after subdivided analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download