INTRODUCTION
MATERIALS AND METHODS
Patients and data acquisition
Ustekinumab treatment and analysis of drug survival
Statistical analysis
RESULTS
Patient characteristics
Table 1
Baseline demographics and characteristics of patients (n=98)
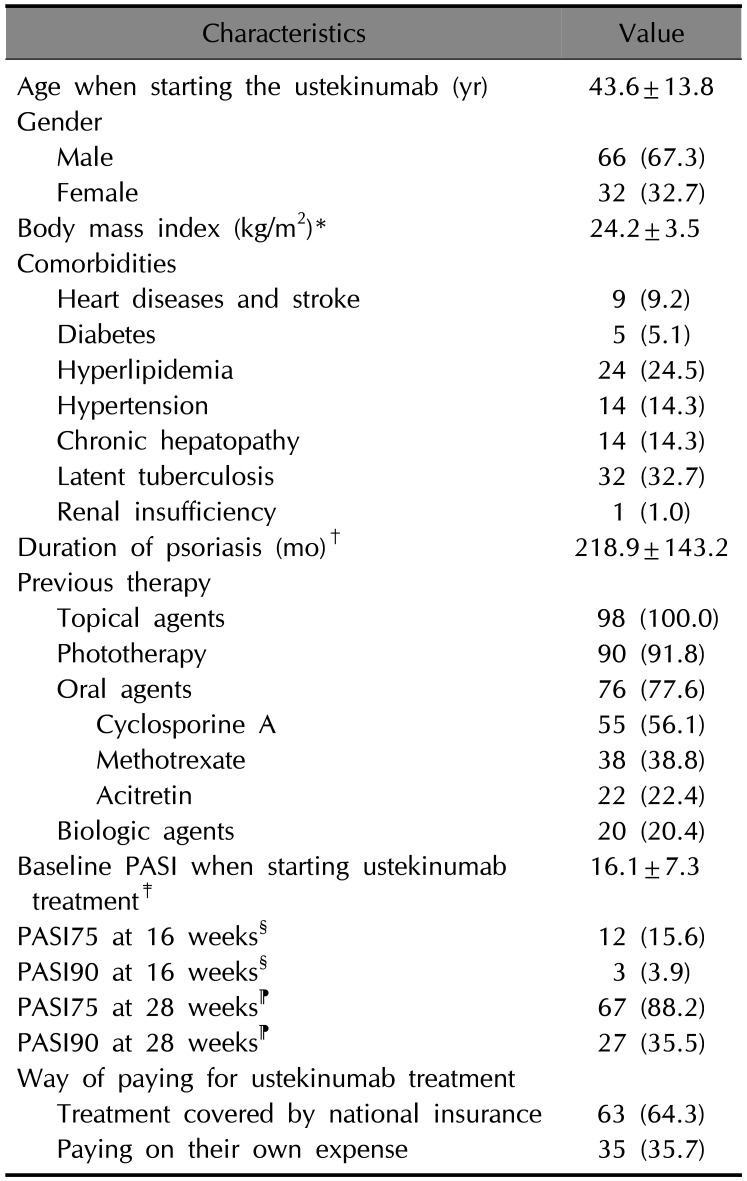
Values are presented as mean±standard deviation or number (%). PASI: Psoriasis Area and Severity Index. *Fifteen patients with unknown weight or height were omitted from the calculation. †Two patients with duration not known were omitted from the calculation. ‡Ten patients with baseline PASI not known were omitted from the calculation. §Twenty-one patients with unknown baseline PASI or PASI at 16 weeks were omitted from the calculation. ⁋Twenty-two patients with unknown baseline PASI or PASI at 28 weeks were omitted from the calculation.
Overall drug survival rate for ustekinumab treatment and factors affecting drug survival
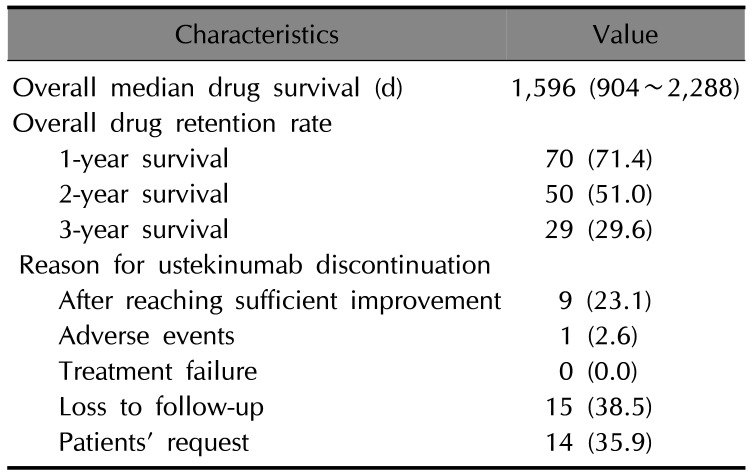
Table 2
Drug survival for ustekinumab treatment (n=98) and the reasons for discontinuation of ustekinumab treatment (n=39)
 | Fig. 1Overall ustekinumab drug survival and comparison of drug survival between treatment covered by insurance and treatment paid at their own expense. Log-rank test revealed significant differences in drug survival between ustekinumab treatment covered by insurance and on their own expenses (p<0.001). |
Table 3
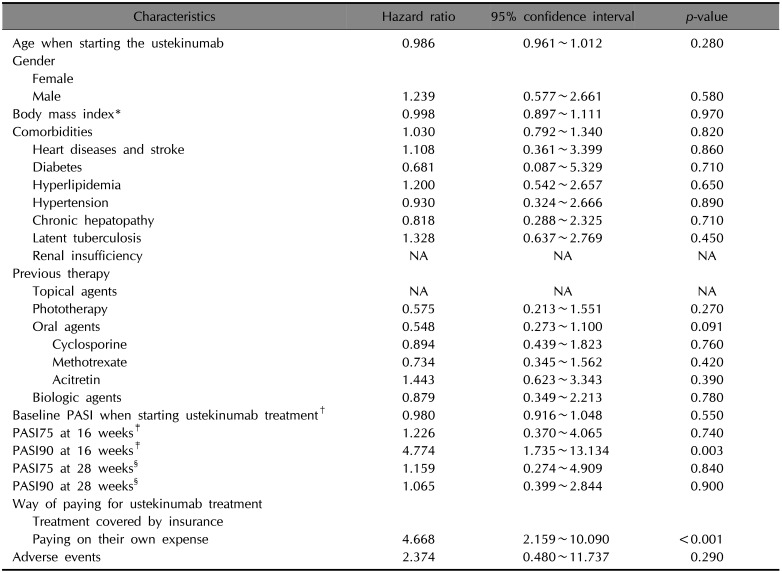
Univariate Cox proportional hazard analysis for ustekinumab treatment (n=98)
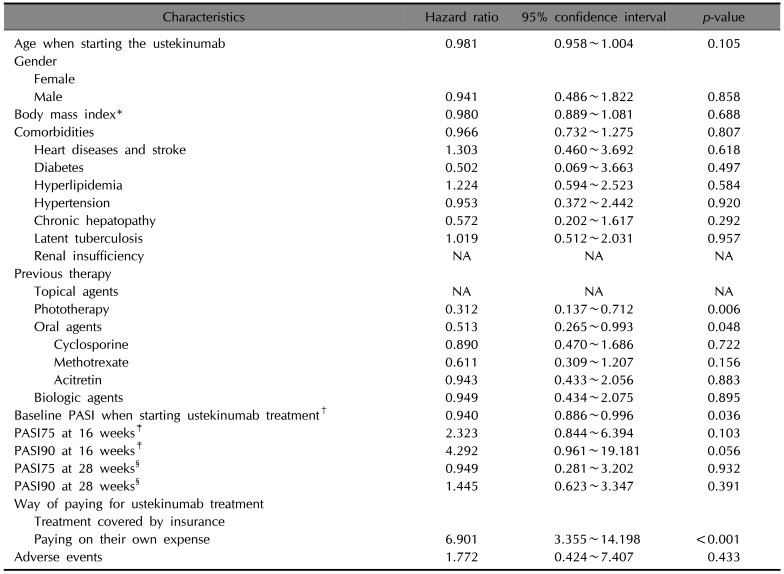
PASI: Psoriasis Area and Severity Index, NA: not applicable. *Fifteen patients with unknown weight or height were omitted from the calculation. †Ten patients with unknown baseline PASI were omitted from the calculation. ‡Twenty-one patients with unknown baseline PASI or PASI at 16 weeks were omitted from the calculation. §Twenty-two patients with unknown baseline PASI or PASI at 28 weeks were omitted from the calculation.
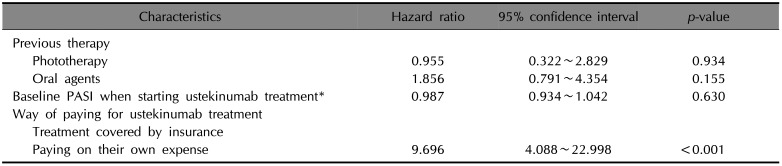
Table 4
Multivariate Cox proportional hazard analysis for ustekinumab treatment (n=98)
Competing risk regression analysis and factors affecting discontinuation of ustekinumab for reasons other than satisfaction
Table 5
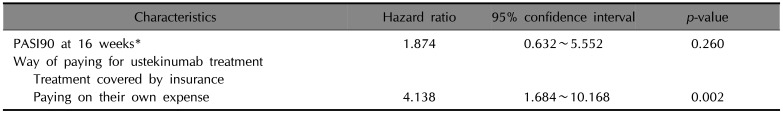
Univariate competing risk regression analysis for ustekinumab treatment (n=98)
PASI: Psoriasis Area and Severity Index, NA: not applicable. *Fifteen patients with unknown weight or height were omitted from the calculation. †Ten patients with unknown baseline PASI were omitted from the calculation. ‡Twenty-one patients with unknown baseline PASI or PASI at 16 weeks were omitted from the calculation. §Twenty-two patients with unknown baseline PASI or PASI at 28 weeks were omitted from the calculation.
Table 6
Multivariate competing risk regression analysis for ustekinumab treatment (n=98)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download