Abstract
Background
Adiponectin, an adipokine secreted from adipocytes, affects energy metabolism and also shows anti-diabetic and anti-inflammatory properties. Recent studies have reported that adiponectin plays a role in regulating skin inflammation.
Objective
This study aimed to investigate the effect of adiponectin on the expression of filaggrin (FLG) in normal human epidermal keratinocytes (NHEKs).
Methods
NHEKs were serum-starved for 6h before being treated with adiponectin. Afterward, cell viability was assessed by MTT assay. We also treated with calcium, interleukin (IL)-4, and IL-13 to provide positive and negative comparative controls, respectively. Gene mRNA expression was quantified using real time reverse transcription polymerase chain reaction, and protein expression was evaluated using Western blot. To evaluate the relationship among mitogen-activated protein kinases (MAPKs), activator protein 1 (AP-1), and FLG, we also treated cells with inhibitors for MAPKs JNK, p38, and ERK1/2.
Adiponectin is an adipokine protein that is primarily secreted from adipocytes1. It is closely related to energy metabolism process and involved in lipid regulation, insulin sensitivity, and cardiovascular functions234. Additionally, adiponectin possesses anti-inflammatory and anti-atherogenic properties56.
There have been several recent studies characterizing the effect of adiponectin treatment on skin. In addition to modulating the proliferation, migration, and cytokine secretion of keratinocytes, adiponectin regulates the cutaneous wound healing process789 and the growth and differentiation of keratinocytes10. Furthermore, adiponectin plays a role in regulating skin inflammation such as psoriasis11,12. Beyond the anti-inflammatory effect of adiponectin on the skin, it may also provide protection against ultraviolet-induced dermal matrix degradation in photoaged human skin1314.
The filaggrin (FLG) protein is known to play a key role in maintaining skin barrier function15. The levels of FLG protein and the products of its breakdown are important for skin barrier function, and common loss-of-function mutations in the FLG gene are the strongest known risk factor for atopic dermatitis (AD)1617.
We previously reported that adiponectin upregulates FLG expression through an SIRT1 (silent mating type information regulation 2 homolog 1)-mediated pathway and suggested that adiponectin might be a promising agent for improving skin barrier function18. This study aimed to explore an additional mechanism by which adiponectin promotes expression of FLG in normal human epidermal keratinocytes (NHEKs).
NHEKs, cell culture media (EpiLife, with calcium), human keratinocyte growth serum, and other cell culture materials were purchased from Gibco BRL, Life Technologies (Grand Island, NY, USA). Recombinant human interleukin (IL)-4 and IL-13 (variant), produced in Escherichia coli, were purchased from Peprotech (Rocky Hill, NJ, USA). Specific antibodies used for Western blot analysis were purchased from Cell Signaling Technology (Danvers, MA, USA). MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) was purchased from Sigma (St. Louis, MO, USA). The pharmacologic inhibitors of c-Jun amino-terminal kinase (JNK) (SP600125), p38 (SB203580), and extracellular signal-regulated kinases 1/2 (ERK1/2) (PD98059) were purchased from Calbiochem (San Diego, CA, USA).
NHEKs were maintained in a 5% CO2 humidified atmosphere at 37℃ in EpiLife media supplemented with calcium. The cells were collected at the second or third passage for further experimental use. Prior to all experiments, cells were conditioned for between 4 and 6 h in serum-free media. Cell viability was measured using MTT reduction assays, as described previously19. Briefly, cells were incubated for at least 24 h in 96-well plates and pre-treated with different concentrations of adiponectin (2.5, 5, 10, and 20 µg/ml) for an additional 24 h, 48 h, or 72 h. Afterward the culture supernatants were removed, and the resulting purple formazan was dissolved in dimethyl sulfoxide20. Absorbance values were measured at 562 nm using a microplate spectrophotometer, SpectraMax340pc (Molecular Devices, Sunnyvale, CA, USA). Cell viability was calculated relative to the absorbance of the adiponectin untreated normal control (NC) group.
Standard procedures were used for all Western blotting analysis. Briefly, cells were lysed in 1% Triton-X radioimmunoprecipitation assay buffer for 1 min. Cellular debris was removed by centrifugation at 13,200 rpm for 15 min. Lysate protein concentration was determined using the bicinchoninic acid assay method. Cell lysates that contained equal amounts of protein (~35 µg of total protein) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and electro-blotted onto nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin and then incubated with the desired primary and secondary antibodies. Protein expression was detected using the EzWestLumi plus system (ATTO, Tokyo, Japan), according to the manufacturer's instructions. Blots were visualized using a ChemiDocTM XRS image analyzer (Bio-Rad, Hercules, CA,USA), and protein expression levels were quantified using ImageJ software and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin.
Total RNA was extracted from keratinocytes using TRIzol® reagent (Welgene, Seoul, Korea) following the manufacturer's recommended protocol. The cDNA synthesized from mRNA was then incubated for an additional 1 h at 42℃. Single-stranded cDNA was amplified by PCR with specific primers for genes encoding FLG, FLG-2, GAPDH, and cyclophilin A (CypA). Initial mRNA quantities were determined after performing real-time PCR using a thermal cycler (Bio-Rad) with SYBR premix Ex Taq (Takara, Shiga, Japan) following the manufacturer's recommended protocol. All measurements include duplicate experiments, and the results were analyzed using the 2−ΔΔCT method. Expression data were normalized to GAPDH and CypA.
Because FLG is involved in the terminal differentiation of keratinocytes to form the cornified cell envelope21, a 1.2 mM concentration of calcium (Ca2+), as a positive control for FLG expression. Previous studies have shown that IL-4 and IL-13 inhibit FLG and FLG-2 gene expression, respectively2223. Recombinant IL-4 (50 ng/ml) and IL-13 (50 ng/ml) were added to the keratinocyte culture media for 5 days in order to provide a negative experimental control for FLG expression.
All in vitro data are presented as the mean±standard deviation (SD). The mean values were calculated based on data from at least three independent replicate experiments that were conducted on separate days using freshly prepared reagents. Data were analyzed using paired t-test. Significant differences were defined at p-value <0.05. All statistical analyses were performed using PASW SPSS ver. 18.0 (IBM Co., Armonk, NY, USA).
Adiponectin induced no cytotoxicity up to a concentration of 10 µg/ml. However, cell viability was significantly reduced when NHEKs were treated with a concentration of 20 µg/ml adiponectin for 24 h (Fig. 1A). Protein and mRNA expression of involucrin and FLG, which are common markers of keratinocyte differentiation, was increased by adiponectin in a dose-dependent manner (Fig. 1B). As such, all subsequent experiments were conducted using a concentration of 10 µg/ml adiponectin.
In the analysis of the transcriptional level of FLG using RT-PCR following a time course of up to 120 h of adiponectin treatment, the relative mRNA level of FLG was significantly higher 72 h and 96 h after the start of adiponectin incubation (Fig. 2A). The relative mRNA level of FLG-2 was significantly higher between 48 h and 72 h after the start of incubation with adiponectin (Fig. 2B). These results suggest that adiponectin simultaneously promotes the transcriptional upregulation of FLG and FLG-2.
Both IL-4 and IL-13 significantly inhibited FLG and FLG-2 gene expression compared with the NC. The simultaneous addition of adiponectin along with IL-4 and IL-13, however, successfully reversed their inhibition of FLG and FLG-2 gene expression (Fig. 2C, D). These results show that adiponectin restores FLG and FLG-2 mRNA expression under normally inhibitory conditions. Interestingly, the simultaneous addition of adiponectin and Ca2+ augmented the inductive action of Ca2+ on FLG and FLG-2 gene expression, demonstrating that adiponectin exerts a synergetic effect with Ca2+ on FLG and FLG-2 mRNA expression. As shown in Fig. 3B, adiponectin and Ca2+ were differently phosphorylation of mitogen-activated protein kinases (MAPKs) protein and the treatment of adiponectin and Ca2+ together was synergetic up-regulated phosphorylation of ERK.
After 10 µg/ml adiponectin treatment, c-Jun and Fra1 proteins were significantly phosphorylated in NHEKs. When NHEKs were treated with adiponectin as well as IL-4 and IL-13, c-Jun (serine 73) and Fra-1 were more phosphorylated compared to their levels after treatment with IL-4 and IL-13 alone (Fig. 3C). This finding suggests that adiponectin may act to regulate the expression of c-Jun and Fra1 proteins, which are both components of the AP-1 transcription factor. Expression of c-Fos protein, however, was not detected (data not shown).
Adiponectin induced the time-dependent phosphorylation of MAPKs (Fig. 3A). When the JNK inhibitor SP600125 (at 10 µM), p38 inhibitor SB203580 (at 10 µM), and ERK inhibitor PD98059 (at 10 µM) were respectively added to NHEKs 1 hour before also adding 10 µg/ml of adiponectin, phosphorylation of JNK/SAPK, p38, and ERK was attenuated (Fig. 4A). The phosphorylation of c-Jun (serine 73) was significantly lower in conditions with the ERK inhibitor, and the phosphorylation of Fra1 was significantly lower in conditions with the JNK and ERK inhibitors (Fig. 4B). The transcriptional levels of FLG and FLG-2 were most effectively attenuated by applying p38 and ERK inhibitors with adiponectin (Fig. 4C, D).
Subcutaneous tissue, including adipose tissue, serves many important functions and critically regulates skin homeostasis and pathophysiology. Accordingly, adipose tissue is sometimes referred to as a cytokine depot, which can then affect the epidermis and dermis via endocrine, paracrine, and autocrine pathways24. Many recent dermatologic studies have suggested that, among the many different adipokines, adiponectin in particular may have beneficial effects on the skin. Normal human keratinocytes express adiponectin receptors AdipoR1 and AdipoR28, and adiponectin is known to regulate the proliferation and migration of keratinocytes9, as well as play a crucial role in the regulation of cutaneous wound healing and skin inflammation1112.
FLG and its high molecular-weight precursor profilaggrin (proFLG) are filament-associated proteins that aggregate keratin fibers in keratinocytes. The cellular processing of proFLG and FLG provides an important material source of natural moisturizing factors (NMF), and multiple proteolytic enzymes have been implicated in their proteolytic processing25. FLG-2 shares numerous properties with FLG, including a closely related structural organization, an identical pattern of expression and localization in the epidermis, and analogous proteolysis processing. As such, it is understood that FLG-2 and FLG have overlapping and synergistic roles driving the formation of the epidermal barrier by degradation to NMF2627.
Our recent study showed that adiponectin upregulates FLG expression through a SIRT1-mediated pathway, suggesting that adiponectin might be a promising agent for enhancing skin barrier function18. We demonstrated that adiponectin downregulates hBD2 mRNA expression through the suppression of p38 and JNK/SAPK MAPK signaling in a previous study28 and performed the present study based on the assumption that adiponectin would have additional signaling mechanisms that promote the expression of FLG.
We first quantified changes in mRNA expression of FLG in NHEKs treated with adiponectin. We then demonstrated that adiponectin exerts a protective effect on the increased level of FLG expression by reversing the inhibition of expression due to treatment with Th2 cytokines IL-4 and IL-13. Our data indicate that adiponectin not only has the ability to promote FLG mRNA expression, but can also prevent inhibition of FLG expression in the Th2 cytokine milieu, such as AD.
AP-1 plays several key roles in the regulation of cell proliferation and differentiation as a regulatory transcription factor29. AP-1 functions as a homodimer or heterodimer made up of FOS (c-Fos, Fra-1, Fra-2) and JUN (c-Jun, JunB, JunD) protein family members30. AP-1 transcriptional factors are already clearly associated with FLG gene expression, as the FLG gene contains AP-1 Jun/Fos family sites in its regulatory regions, and a high basal level of FLG gene expression in keratinocytes depends on the AP-1 component of the c-Jun/c-Fos heterodimers313233. Our results suggest that adiponectin regulates the activity of critical AP-1 components. We showed that the activity of c-Jun and Fra1 significantly increased after adiponectin treatment, and the effect was suppressed when MAPK signaling was inhibited by p38, JNK, and ERK inhibitors. Additionally, the effect of adiponectin on FLG expression was blocked by additional treatment with p38 and ERK inhibitors. Together, these results suggest that adiponectin stimulates FLG expression via a mechanism that depends on AP-1 transcriptional factors and MAPK signaling.
In addition, adiponectin exerted a synergetic effect with calcium on the induction of FLG expression. Accordingly, adiponectin might promote the differentiation of keratinocytes by affecting the level of intracellular calcium. The influence on differentiation could be considered one of the mechanisms associating adiponectin and FLG expression.
In conclusion, our findings suggest that adiponectin acts to regulate FLG expression. The present study demonstrated for the first time that adiponectin plays a role in regulating the expression and processing of FLG in NHEKs. Therefore, adiponectin may be a potential therapeutic agent to control skin diseases that alter the skin barrier.
Figures and Tables
 | Fig. 1The effect of adiponectin on the differentiation of keratinocytes. (A) Keratinocyte cell viability after adiponectin treatment at 24 h, 48 h, and 72 h. Cells were serum-starved for 6 h before treatment with adiponectin (2.5, 5, 10, and 20 µg/ml). Cell viability was assessed by MTT assay and expressed as a percentage of the control (untreated) group. (B) Protein and mRNA expression of keratinocyte differentiation markers. Cells were treated with adiponectin (5, 10, and 20 µg/ml) for 72 h. Protein and mRNA expression was analyzed by Western blot and reverse transcription-polymerase chain reaction, respectively. Data are presented as the mean±standard deviation of three independent replicate experiments (n=3). NS: no significance, NC: normal control group, GAPDH: glyceraldehyde 3-phosphate dehydrogenase, FLG, filaggrin. **p<0.005, control vs. adiponectin treatment group. |
 | Fig. 2Adiponectin induces filaggrin (FLG) and FLG-2 mRNA expression. The time dependent relative mRNA expression after adiponectin treated of (A) FLG and (B) FLG-2 was analyzed by real time reverse transcription-polymerase chain reaction. Data are represented in graphical form and show the fold change compared to normal control (NC) cells of the 1 h incubation group. The protective effect of adiponectin on (C) FLG and (D) FLG-2 mRNA expression under normally inhibitory treatment with Th2 cytokines interleukin (IL)-4 and IL-13. Calcium (1.2 mM) was used as a positive control for FLG expression. Data are presented in graphical form and show the fold change compared to NC cells. CypA expression levels were used as an internal control. Data are presented as the mean±standard deviation of three independent replicate experiments (n=3). (A, B) *p<0.05, **p<0.005, NC group vs. adiponectin-treated group. (C, D) *p<0.05; **p<0.005 vs. NC; #p<0.05, ##p<0.005 vs. IL-4- and IL-13-treated group. |
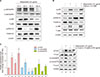 | Fig. 3Adiponectin induced phosphorylation of mitogen-activated protein kinases (MAPKs) and activator protein 1. (A) The time-dependent phosphorylation of MAPKs was induced by adiponectin treatment (10 µg/ml). (B) Adiponectin and calcium (1.2 mM) differently phosphorylation of MAPKs proteins. Adiponectin regulated the phosphorylation of (C) Fra-1 and c-Jun protein expression. Protein and mRNA expression levels were analyzed by Western blot and reverse transcription-polymerase chain reaction, respectively. Data are presented in graphical form and show the fold change compared with sub-confluent normal control (NC) cells. Data are presented as the mean±standard deviation of three independent replicate experiments (n=3). *p<0.05, **p<0.005 vs. NC group; #p<0.05 vs. IL-4- and IL-13-treated group. |
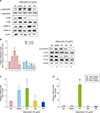 | Fig. 4Adiponectin regulation of filaggrin (FLG) mRNA expression depends on the activator protein 1 components c-Jun and fos related antigen-1 (Fra-1) and mitogen-activated protein kinase (MAPK) signaling. (A) MAPK inhibitors attenuated the phosphorylation of JNK/SAPK, ERK and p38. The relative (B) protein expression of Fra-1 and cJun and mRNA expression of (C) FLG and (D) FLG-2 after treatment with adiponectin and MAPK inhibitors. Normal human epidermal keratinocytes were treated with MAPK inhibitors SP600125, SB203580, and PD98059 before adiponectin treatment. Protein and mRNA expression levels were analyzed by Western blot and reverse transcription-polymerase chain reaction, respectively. Data are presented in graphical form and show the fold change compared with sub-confluent normal control (NC) cells. Data are presented as the mean±standard deviation of three independent replicate experiments (n=3). DMSO: used as vehicle, dimethyl sulfoxide, SP: SP600125, JNK inhibitor, SB: SB203580, p38 inhibitor, PD: PD98059, ERK inhibitor. *p<0.05, **p<0.005 vs. NC group; &p<0.05, &&p<0.005 vs. adiponectin-treated group. |
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HN14C0095).
References
1. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995; 270:26746–26749.

2. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001; 86:1930–1935.

3. Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, et al. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab. 2005; 90:3731–3737.

4. Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004; 89:2563–2568.

5. Magge SN, Stettler N, Koren D, Levitt Katz LE, Gallagher PR, Mohler ER 3rd, et al. Adiponectin is associated with favorable lipoprotein profile, independent of BMI and insulin resistance, in adolescents. J Clin Endocrinol Metab. 2011; 96:1549–1554.

6. Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000; 102:1296–1301.

7. Salathia NS, Shi J, Zhang J, Glynne RJ. An in vivo screen of secreted proteins identifies adiponectin as a regulator of murine cutaneous wound healing. J Invest Dermatol. 2013; 133:812–821.

8. Shibata S, Tada Y, Asano Y, Hau CS, Kato T, Saeki H, et al. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol. 2012; 189:3231–3241.

9. Takahashi H, Honma M, Ishida-Yamamoto A, Iizuka H. Adiponectin and leptin modulate cell proliferation and cytokine secretion of normal human keratinocytes and T lymphocytes. J Dermatol Sci. 2010; 59:143–145.

10. Kawai K, Kageyama A, Tsumano T, Nishimoto S, Fukuda K, Yokoyama S, et al. Effects of adiponectin on growth and differentiation of human keratinocytes--implication of impaired wound healing in diabetes. Biochem Biophys Res Commun. 2008; 374:269–273.

11. Shibata S, Tada Y, Hau CS, Mitsui A, Kamata M, Asano Y, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat Commun. 2015; 6:7687.

12. Wolk K, Sabat R. Adipokines in psoriasis: an important link between skin inflammation and metabolic alterations. Rev Endocr Metab Disord. 2016; 17:305–317.

13. Fang CL, Huang LH, Tsai HY, Chang HI. Dermal lipogenesis inhibits adiponectin production in human dermal fibroblasts while exogenous adiponectin administration prevents against UVA-induced dermal matrix degradation in human skin. Int J Mol Sci. 2016; 17:pii:E1129.

14. Kim EJ, Kim YK, Kim MK, Kim S, Kim JY, Lee DH, et al. UV-induced inhibition of adipokine production in subcutaneous fat aggravates dermal matrix degradation in human skin. Sci Rep. 2016; 6:25616.

16. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006; 38:441–446.

17. Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014; 134:792–799.

18. Jin T, Park KY, Seo SJ. Adiponectin upregulates filaggrin expression via SIRT1-mediated signaling in human normal keratinocytes. Ann Dermatol. 2017; 29:407–413.

19. Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989; 119:203–210.

20. Rajapakse N, Kim MM, Mendis E, Kim SK. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology. 2008; 123:348–357.

21. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005; 6:328–340.

22. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009; 124:R7–R12.

23. Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Méchin MC, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013; 131:1094–1102.

24. Paus R, Klein J, Permana PA, Owecki M, Chaldakov GN, Böhm M, et al. What are subcutaneous adipocytes really good for...? Exp Dermatol. 2007; 16:45–47.
25. Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009; 122:1285–1294.

26. Hsu CY, Henry J, Raymond AA, Méchin MC, Pendaries V, Nassar D, et al. Deimination of human filaggrin-2 promotes its proteolysis by calpain 1. J Biol Chem. 2011; 286:23222–23233.

27. Wu Z, Hansmann B, Meyer-Hoffert U, Gläser R, Schröder JM. Molecular identification and expression analysis of filaggrin-2, a member of the S100 fused-type protein family. PLoS One. 2009; 4:e5227.

28. Kim M, Park KY, Lee MK, Jin T, Seo SJ. Adiponectin suppresses UVB-induced premature senescence and hBD2 overexpression in human keratinocytes. PLoS One. 2016; 11:e0161247.

29. Mehic D, Bakiri L, Ghannadan M, Wagner EF, Tschachler E. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J Invest Dermatol. 2005; 124:212–220.

30. Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004; 117:5965–5973.

31. DiSepio D, Bickenbach JR, Longley MA, Bundman DS, Rothnagel JA, Roop DR. Characterization of loricrin regulation in vitro and in transgenic mice. Differentiation. 1999; 64:225–235.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download