Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal tract and occur most frequently in the stomach. The liver is the most common metastatic site of a GIST, and spontaneous rupture of the hepatic metastasis of a malignant gastric GIST is rare. We report the case of a 70-year-old man who presented with sudden right lower quadrant abdominal pain and was diagnosed with a spontaneously ruptured hepatic metastasis of a malignant gastric GIST. The patient was successfully managed with transcatheter arterial embolization of the hepatic artery.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and occur most frequently in the stomach, small bowel, esophagus, and omentum.12 The liver is the most common metastatic site of GISTs.123 The spontaneous rupture of the hepatic metastasis of a GIST is extremely rare.1245 We report a case with a spontaneously ruptured hepatic metastasis of a malignant gastric GIST, which was successfully treated with transcatheter arterial embolization (TAE) of the hepatic artery.
A 70-year-old man presented to the emergency department with sudden right lower quadrant abdominal pain radiating to the shoulder for 1 day. Abdominal pain with a squeezing nature and a numerical rating score of 9 continued from the onset to the arrival at the emergency department. On admission, physical examination revealed blood pressure of 136/59 mmHg, a pulse rate of 75/min, body temperature of 36.5℃, and tenderness and rebound tenderness in the right lower quadrant abdomen. The bowel sound was normative and there was no shifting dullness. Other physical findings were normal. The laboratory examination results were as follows: white blood cells 6,380/mm3; hemoglobin 11.0 g/dL; platelet count 119,000/mm3; PT INR 0.99; aPTT 29.7 seconds; AST 46 IU/L; ALT 32 IU/L; ALP 81 IU/L; AFP 2.26 ng/mL; HBsAg (−), anti-HBs (−), and anti-HCV (−).
At presentation, abdominal multidetector CT showed a hematoma in the subphrenic and subhepatic spaces. Multiple low-density nodular lesions were identified in both lobes of the liver. A round mass showing exophytic growth was noted in the posterior-inferior segment of the liver. Capsular disruption was noted on the inferomedial wall of the mass lesion combined with the hemoperitoneum around the mass lesion (Fig. 1). Angiography and embolization were determined to prevent worsening of intraperitoneal hemorrhage and improve intractable abdominal pain. Emergent angiography of the common hepatic artery revealed a round hypervascular mass lesion in the inferior angle of the liver supplied by a branch from the posterior segmental artery of the liver (Fig. 2A). After superselection of the feeding branch of the hepatic artery with a microcatheter (Asahi Tellus; ASAHI INTECC Co., Ltd., Seto, Japan) and micro-guidewire (Asahi Meister; ASAHI INTECC Co., Ltd.), the feeding branch was embolized with polyvinyl alcohol particles (300-500 µm in size; Bearing nsPVA; Merit Medical System, South Jordan, UT, USA). Post-embolization angiography showed no further visualization of the hypervascular mass lesion in the liver (Fig. 2B). The vital signs were stable and the abdominal pain was regressed.
The patient had undergone laparoscopic partial gastrectomy for a 3.5-cm-sized c-KIT-positive GIST of the stomach with a mitotic count of 29 per 50 high-power fields three years prior. Three months later, the contrast enhance CT revealed a new small low-density (8 mm in diameter) nodule in the lateral segment of the liver. This low-density nodule showed an increased size (20 mm in diameter) on CT 2 months later. This lesion was considered a metastatic lesion of the gastric GIST that was resected 5 months prior because the low-density nodular lesion within the liver newly appeared after surgery and showed an increase in size on follow-up CT. Palliative chemotherapy with imatinib was performed for 2.5 years thereafter. After the initiation of chemotherapy, this nodular lesion of the liver showed a decrease in size (2 cm → 1.5 cm) at the 2-month follow-up CT study. During chemotherapy for 2.5 years, the liver nodule showed a wax-and-wane pattern in size, suggesting a response to imatinib.6 A local recurrence lesion at the gastric resection site was found to have newly developed when chemotherapy was performed for 2.5 years and was treated with additional wedge resection. Metastatic lesion in the lateral segment of the liver was still visible and was treated with lateral segmentectomy of the liver at the same time. The recurred lesion of the stomach resection margin was 3.5 cm in size with a mitotic count of more than 100 per 50 high power fields and c-KIT positivity. The nodular lesion of the liver showed c-KIT positivity. Because the primary GIST was in the high-risk category, the patient underwent adjuvant chemotherapy with imatinib. At 7 months after chemotherapy, multiple metastatic lesions were noted in both lobes of the liver. The patient was admitted to hospital due to abdominal pain radiating to the shoulder and lasting for 1 day at 8 months after chemotherapy.
The patient received radiotherapy for 2 weeks with a total dose of 40 Gy in 16 fractions to the ruptured metastatic lesion of the liver to relieve residual abdominal pain, which showed a numerical rating score of 3 even after embolization. The patient was discharged 3 weeks after embolization and had an uneventful course over the subsequent 6-month period while undergoing chemotherapy using imatinib.
GISTs are the most common mesenchymal tumors of the gastrointestinal tract. Since 1983, they have been differentiated from leiomyomas, leiomyoblastomas, leiomyosarcomas, and other mesenchymal tumors by their electron microscopic and immunohistochemical characteristics.2 The major diagnostic criterion of GISTs is the expression of c-KIT (CD 117 antigen), a tyrosine-kinase growth factor receptor that differentiates them from true leiomyomas, leiomyoblastomas, and neurofibromas.7 GISTs are the most common mesenchymal tumors of the gastrointestinal tract and may occur from the esophagus to the anus. They may also occur primarily in the omentum, mesentery, and retroperitoneum.7 Malignant GISTs commonly metastasize to the liver or peritoneum. The liver is the most common metastatic site at both initial presentation and recurrence of the disease.3
Benign and malignant tumors of the liver are among the most common causes of spontaneous hepatic rupture. Hepatocellular carcinoma is the leading cause among malignant tumors, and hepatocellular adenoma is the leading cause among benign tumors.58 Hemoperitoneum secondary to spontaneous rupture of hepatic metastases from primary tumors of the lung, pancreas, stomach, kidney, breast, prostate, testicle, gallbladder, skin (melanoma), nasopharyngeal cancer, choriocarcinoma, and lymphoma has been reported.8 However, the spontaneous rupture of hepatic metastases from GISTs and the hemoperitoneum is rarely reported.1245
The precise cause of the spontaneous rupture of a GIST or metastatic GIST is unknown. The rupture may originate at a wall of a mass that is weakened by cystic or hemorrhagic degeneration.1 Exophytic growth or subcapsular location of the primary or metastatic tumors of the liver is known to be related to spontaneous rupture.128 In our case, the spontaneously ruptured metastatic lesion of the liver showed exophytic growth and subcapsular location in the liver. In spontaneously ruptured hepatocellular carcinoma, rapid growth of the tumor and necrosis, splitting of the overlying normal liver parenchyma, erosion of a vessel, occlusion of the hepatic veins by a tumor thrombus, and coagulopathy are regarded as possible explanations.9
The clinical presentation of spontaneous ruptures of hepatic metastases ranges from asymptomatic to severe abdominal pain and rapid hypovolemic shock.12458 In some cases, these symptoms are the first manifestation of an underlying liver disease, often encountered in an emergency setting.2 The diagnosis of hemorrhagic metastasis is suggested if blood is identified in one or more liver lesions in a patient with known hepatic metastases or a known primary tumor elsewhere.8 If the hemorrhage is severe, a subcapsular hematoma or hemoperitoneum may also be noted.28 The presence and extent of intrahepatic or subcapsular hematoma and hemoperitoneum can be easily identified using CT and may appear as a hyperattenuating mass or a mass with high-density material or hyperdense ascites.18 In GIST, the presence of ascites is uncommon, even in stages of advanced peritoneal dissemination. Therefore, when it is present, the possibility of rupture should be considered.2 Capsular disruption of the metastatic lesion of the liver, associated with hemoperitoneum, may be demonstrated in CT.1 Spontaneous rupture of a hepatic lesion is a relatively rare acute complication of several hepatic pathologies. Its sudden appearance and potentially life-threatening outcome make it an important diagnostic and therapeutic challenge. Because abdominal pain is nonspecific, a high level of alertness is necessary to reach the timely diagnosis of a spontaneous rupture of a hepatic lesion.5
Conservative treatment, TAE, and surgical management have been performed for ruptured primary or metastatic lesions of the liver.1259 The treatment method used for patients with a spontaneously ruptured hepatic mass should be based on the patient's condition, cause of the rupture, and characteristics of the hepatic lesion.5 The primary aim of managing a spontaneously ruptured metastatic lesion of a GIST would be to control hemorrhage.12 TAE is considered the most effective treatment to stop hemorrhage from a ruptured primary or metastatic tumor of the liver.18910 TAE has been increasingly used for ruptured primary or metastatic lesions of the liver and is an effective and less invasive treatment to achieve immediate hemostasis.19 This patient showed intractable abdominal pain requiring further management although he received conservative management. Angiography and embolization were performed to control hemorrhage and hemoperitoneum, while the vital signs of the patient were stable. Further external radiotherapy was performed to manage abdominal pain persisting after embolization of the hepatic artery supplying the ruptured metastatic lesion.
In conclusion, spontaneous rupture of hepatic metastases of gastric GISTs that present as abdominal pain and hemoperitoneum is rare, for which a high index of suspicion is necessary for timely diagnosis and treatment. Imaging studies such as CT can be an important tool to diagnose spontaneous rupture and hemoperitoneum and to differentiate this medical condition from others. TAE may be an optional treatment method to achieve hemostasis and control symptoms.
Figures and Tables
 | Fig. 1Contrast-enhanced abdominal CT image of a 70-year-old man with spontaneous rupture of a hepatic metastasis from a gastric GIST. Axial (A) and coronal (B) CT images show a round-shaped, low-density mass (asterisk) in the inferior angle of the liver. This metastatic lesion located in the subcapsular area shows an irregular and broken inferior-medial margin (long arrows) of the metastatic lesion, suggesting rupture. Hemoperitoneum is noted in the subhepatic (short arrows) and subphrenic spaces (empty arrows). CT, computed tomography; GIST, gastrointestinal stromal tumor. |
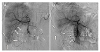 | Fig. 2Angiography of the common hepatic artery of a 70-year-old man. (A) Angiography of the common hepatic artery shows a round-shaped, hypervascular mass (short arrows) in the inferior angle of the liver supplied by the posterior-inferior segmental hepatic artery (empty arrows). (B) Post-embolization angiography of the common hepatic artery revealed a disappeared and obstructed hypervascular mass (short arrows) supplying the hepatic artery. |
References
1. Yoon JH. A spontaneously ruptured hepatic metastasis from a gastric gastrointestinal stromal tumor that presented as hemoperitoneum. J Investig Med High Impact Case Rep. 2013; 1:2324709613512475.

2. Cegarra-Navarro MF, de la Calle MA, Girela-Baena E, García-Santos JM, Lloret-Estañ F, de Andrés EP. Ruptured gastrointestinal stromal tumors: radiologic findings in six cases. Abdom Imaging. 2005; 30:535–542.

3. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000; 231:51–58.
4. Suzuki K, Kaneko G, Kubota K, et al. Malignant tumor, of the gastrointestinal stromal tumor type, in the greater omentum. J Gastroenterol. 2003; 38:985–988.

5. Maoz D, Sharon E, Chen Y, Grief F. Spontaneous hepatic rupture: 13-year experience of a single center. Eur J Gastroenterol Hepatol. 2010; 22:997–1000.

6. Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics. 2006; 26:481–495.

7. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003; 23:283–304. 456quiz 532.
8. Casillas VJ, Amendola MA, Gascue A, Pinnar N, Levi JU, Perez JM. Imaging of nontraumatic hemorrhagic hepatic lesions. Radiographics. 2000; 20:367–378.

9. Kim JY, Lee JS, Oh DH, Yim YH, Lee HK. Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2012; 24:640–645.

10. Tekin F, Ozutemiz O, Parýldar M. Successful treatment for the rupture of a hepatic metastasis of GIST. J Gastrointestin Liver Dis. 2006; 15:419–420.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download