Abstract
Splenic abscess is a rare disease that generally occurs in immunocompromised patients. It is difficult to distinguish between splenic abscesses and cysts using imaging studies, especially if they are asymptomatic. A 50-year-old asymptomatic man who had received steroid therapy for underlying rheumatoid arthritis was referred to a university hospital due to presence of several splenic cysts, with the largest being 3.5 cm in diameter. Percutaneous aspiration was performed, and fluid analysis showed cysts infected by extended-spectrum, beta-lactamase-producing Escherichia coli. The patient was treated with ertapenem for four weeks, and the lesion disappeared on follow-up imaging studies. Splenic abscess should be included as a differential diagnosis of splenic cystic lesions in immunocompromised patients.
Splenic abscess is a rare disease, with a rate of 0.05–0.7% in autopsy studies.1 Splenic abscesses generally occur in patients with immunodeficiency syndrome, chemotherapy, cancer, bone marrow and solid organ transplantation, long-term steroid use, monoclonal antibody use, use of immunosuppressive medications, and other conditions, including diabetes mellitus and alcoholism.2 The most common symptoms of splenic abscess are fever (85.1%), left upper quadrant pain (43.3%), and diffuse abdominal pain (14.9%). Physical examination often reveals splenomegaly (50.7%), left upper quadrant tenderness (44.7%), generalized abdominal tenderness (13.4%), and leukocytosis (70.1%).1 The incidence of splenic abscess is thought to be increasing, which may be attributed to increased prevalence of immunodeficiencies, such as chronic corticosteroid use, aggressive chemotherapy, or organ transplantation.3 The management of splenic abscess usually gives good results and is based on medical therapy with antibiotics and splenectomy or percutaneous drainage.4 Successful treatment depends on patient's comorbidities, general condition, and the size and topography of abscess.5 Empiric broad-spectrum antibiotic therapy plays a primary role in the management of splenic abscesses. Percutaneous drainage has been accepted as an effective and less invasive treatment compared with surgical treatment in some patients because such drainage preserves the spleen and avoids the risk of overwhelming postsplenectomy sepsis.
A 50-year-old man was referred to a university hospital for further evaluation of splenic cysts. He had underlying rheumatoid arthritis that had been treated with methotrexate, sulfasalazine, hydroxychloroquine, methylprednisolone, and non-steroidal anti-inflammatory drugs for 10 years. He had a history of previous cholecystectomy, which had been performed 8 years ago. He had no neurological, rheumatological, ophthalmologic, or dermatological symptoms. Physical examination revealed normal blood pressure and body temperature, no abdominal tenderness, and no hepatosplenomegaly.
Laboratory examination indicated no leucocytosis (white blood count of 7.43 103/µL), elevated erythrocyte sedimentation rate of 15 mm/h, and decreased albumin level of 2.31 g/dL. Abdominal-pelvic computed tomography, which was taken at the referring hospital, revealed a 3.5 cm cystic lesion with an internal debris in the spleen anterior pole with multifocal lenticular shaped cystic changes in the spleen periphery. The primary differential diagnosis was hemorrhagic cystic degeneration as a sequalae of focal splenic infarct with cystic lymphangioma. However, due to poor image quality, and the presence of internal debris and periphery lesions, liver magnetic resonance imaging was performed for differential diagnosis and showed a 3.5 cm well-capsulized cystic lesion with internal debris and other smaller cystic lesions in the spleen (Fig. 1). The main differential diagnosis using the MRI were cystic lymphangioma, cystic degeneration of other unusual splenic lesion, or splenic infarct sequalae.
However, given that our patient was immunocompromised, there was a small possibility of splenic abscess. Therefore, the patient underwent ultrasound-guided and fluoroscopic percutaneous catheter drainage for diagnosis and treatment of splenic cyst. Aspiration fluid analysis revealed a turbid, neutrophil-dominant fluid. Our patient developed high fever 12 hours after percutaneous catheter drainage. Blood cultures were performed, and intravenous ceftriaxone and metronidazole were administered. Extended-spectrum beta-lactamase-producing Escherichia coli was cultured in the aspiration fluid, and ertapenem was administered for a period of 4 weeks. Follow-up abdominal-pelvic computed tomography showed that splenic infected cysts nearly disappeared after 4 weeks (Fig. 2).
Splenic abscess is considered an uncommon entity that has recently been increasing in frequency. This is likely due to increased number of immunocompromised and cancer patients.6 Splenic abscesses are often misdiagnosed because the signs and symptoms are nonspecific as in this case, which had no general symptoms like fever or abdominal pain. Immunocompromised states may increase the risk of infection and can lead to disease progression, sepsis, and even death.
In the case of splenic cystic lesions, management is based upon both clinical history and imaging studies. Usually, if cystic lesion with benign imaging characteristics (e.g., either a cyst or a homogenous, low-attenuated lesion with no enhancement and smooth margins) is detected in an asymptomatic patient, further evaluation and follow-up imaging are not usually required. However, if CT findings are inconclusive, MRI can be performed with follow-up imaging at 6–12 months after the initial assessment of growth.7 In cases where differential diagnosis for infection or neoplasm are needed, needle aspiration may be considered. Although needle aspiration of the spleen is reluctantly performed, due to the perceived risk of bleeding and difficulty of access, it has been shown to be relatively safe with high specificity.8 In immunocompromised cases, such as ours, either the close, short-term clinical follow-up with imaging or diagnostic needle aspiration should be considered. Splenic abscesses are usually not diagnosed by radiologic findings, but in conjunction with clinical presentations. Isolated splenic abscesses are rare and more commonly found with concomitant hepatic abscesses, which were absent in our patient. CT findings of splenic abscess usually show hypoattenuation of the nodules, suggesting necrosis. There may also be wedge-shaped, low attenuation lesions representing infarcts due to septic emboli. When abscesses become organized and encapsulated, there may be peripheral enhancement of postcontrast images with enhancement of septations,910 which were not found in this case. Infectious lesions are similar to simple cysts or cystic lymphangioma on MRI as they are typically hypo to isointense on T1 weighted imaging and hyperintense on T2 weighted imaging.9 However, infectious lesions or cystic lymphangiomas differ from simple cysts as they are sometimes presented with possible capsule or septation enhancement.11
Organisms associated with splenic abscesses are gram-positive cocci (Streptococci, Staphylococci), gram-negative bacilli (Escherichia coli, Klebsiella pneumoniae), anaerobes, and fungi. Klebsiella pneumoniae and Escherichia coli have been reported to occur frequently in Southeast Asia.12 Considering the increase in extended-spectrum beta-lactamases-producing microbes in recent years, as well as the fact that these usually occur in immunocompromised patients, carbapenems (imipenem, meropenem, doripenem, and ertapenem) should be considered as the first-line treatment.13
Successful immune suppression may prevent an upregulated host response to serious infections and lead to sepsis.14 In a case without septic conditions, appropriate treatments, such as percutaneous drainage and antibiotics can treat splenic abscess concurrently without interruption of disease- modifying antirheumatic drugs.
The number of immunocompromised patients is increasing, and their symptoms may be nonspecific. Furthermore, radiological findings often have substantial overlap, which precludes a specific diagnosis of simple cyst or abscess on the basis of imaging findings alone.11 Splenic abscess should be considered in the differential diagnosis of splenic cystic lesions in immunocompromised patients, especially in lesions accompanied by internal debris or peripheral splenic infarcts.
Figures and Tables
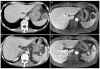 | Fig. 1APCT and MRI showed that 3.5 cm well-capsulized cystic lesion with internal debris in the spleen (A, B) and multifocal lenticular shaped and cystic changes in the periphery of the spleen (C, D). APCT, abdominal-pelvic computed tomography; MRI, magnetic resonance imaging. |
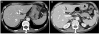 | Fig. 2APCT after one month's treatment showed nearly complete resolution of previous 3.6 cm cystic lesion in the spleen anterior pole, except a tiny 3 mm sized remnant hypodense lesion (A, arrow) and complete disappearance of peripherally multi-located low attenuation in the spleen (B). APCT, abdominal-pelvic computed tomography. |
References
1. Chou YH, Hsu CC, Tiu CM, Chang T. Splenic abscess: sonographic diagnosis and percutaneous drainage or aspiration. Gastrointest Radiol. 1992; 17:262–266.

2. Ng KK, Lee TY, Wan YL, et al. Splenic abscess: diagnosis and management. Hepatogastroenterology. 2002; 49:567–571.
3. Green BT. Splenic abscess: report of six cases and review of the literature. Am Surg. 2001; 67:80–85.
4. de Bree E, Tsiftsis D, Christodoulakis M, Harocopos G, Schoretsanitis G, Melissas J. Splenic abscess: a diagnostic and therapeutic challenge. Acta Chir Belg. 1998; 98:199–202.
5. Schiavo L, Scalera G, De Sena G, Ciorra FR, Pagliano P, Barbarisi A. Nonsurgical management of multiple splenic abscesses in an obese patient that underwent laparoscopic sleeve gastrectomy: case report and review of literature. Clin Case Rep. 2015; 3:870–874.

6. Tung CC, Chen FC, Lo CJ. Splenic abscess: an easily overlooked disease? Am Surg. 2006; 72:322–325.

7. Heller MT, Harisinghani M, Neitlich JD, Yeghiayan P, Berland LL. Managing incidental findings on abdominal and pelvic CT and MRI, part 3: white paper of the ACR incidental findings committee II on splenic and nodal findings. J Am Coll Radiol. 2013; 10:833–839.

8. Keogan MT, Freed KS, Paulson EK, Nelson RC, Dodd LG. Imaging-guided percutaneous biopsy of focal splenic lesions: update on safety and effectiveness. AJR Am J Roentgenol. 1999; 172:933–937.

9. Gaetke-Udager K, Wasnik AP, Kaza RK, et al. Multimodality imaging of splenic lesions and the role of non-vascular, image-guided intervention. Abdom Imaging. 2014; 39:570–587.

10. Ricci ZJ, Oh SK, Chernyak V, et al. Improving diagnosis of atraumatic splenic lesions, part I: nonneoplastic lesions. Clin Imaging. 2016; 40:769–779.

11. Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics. 1996; 16:107–129.

12. Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011; 52:288–292.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download