Abstract
Background/Aims
Direct-acting antiviral (DAA) therapy has been shown to achieve a high rate of sustained virologic response (SVR) and favorable outcomes in chronic hepatitis C (CHC) patients. We investigated the virologic response and its clinical impact in CHC patients.
Methods
CHC patients with compensated liver function treated with DAAs between 2016 and 2017 were included for retrospective analysis. We analyzed baseline characteristics and virologic and biochemical responses at on-treatment 4 weeks, end of treatment, and posttreatment 12 weeks. Fibrosis was measured as liver stiffness measurement by transient elastography (FibroScan). Adverse events were monitored during the treatment period.
Results
A total of 135 patients (61.5% with genotype [GT] 1b and 38.5% with GT 2a) were enrolled 47.4% were male, 79.3% were treatment naive, and 30.4% had cirrhosis. SVR 12 was observed in 97.6% (81/83) in the GT 1b and 98.1% (51/52) in the GT 2a; treatment with daclatasvir+asunaprevir was the most commonly used in GT 1b (55/83), and sofosbuvir+ribavirin was the most commonly used in GT 2a (49/52). The median change of liver stiffness measurement at two time points using the signed rank test was −3.2 kPa in patients who underwent transient elastography before treatment and at SVR 12 (n=25). The most common adverse events were anemia, dyspepsia, and insomnia. One GT 2a patient treated with sofosbuvir+ribavirin stopped the treatment at 8 weeks due to symptomatic bradyarrhythmia; however, he recovered spontaneously and achieved SVR 12.
Go to : 
References
1. Abad S, Vega A, Rincón D, et al. Effectiveness of direct-acting anti-virals in hepatitis C virus infection in haemodialysis patients. Nefrologia. 2017; 37:158–163.

2. Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009; 49:1335–1374.

3. Douhara A, Ogawa H, Nakatani S, et al. Efficacy and HCC development after DAA therapy for patients with chronic hepatitis C: a single center retrospective cohort study. Hepatoma Res. 2017; 3:215–220.

4. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002; 347:975–982.

5. Russo MW, Goldsweig CD, Jacobson IM, Brown RS Jr. Interferon monotherapy for dialysis patients with chronic hepatitis C: an analysis of the literature on efficacy and safety. Am J Gastroenterol. 2003; 98:1610–1615.

6. Manns M, Pol S, Jacobson IM, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014; 384:1597–1605.

7. Reddy KR, Bourlière M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology. 2015; 62:79–86.

8. Welzel TM, Asselah T, Dumas EO, et al. Ombitasvir, paritaprevir, and ritonavir plus dasabuvir for 8 weeks in previously untreated patients with hepatitis C virus genotype 1b infection without cirrhosis (GARNET): a single-arm, open-label, phase 3b trial. Lancet Gastroenterol Hepatol. 2017; 2:494–500.

9. Bourlière M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous pro-tease-inhibitor therapy: a randomized, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis. 2015; 15:397–404.
10. Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014; 59:2083–2091.

11. Wei L, Zhang M, Xu M, et al. A phase 3, open-label study of daclatasvir plus asunaprevir in Asian patients with chronic hepatitis C virus genotype 1b infection who are ineligible for or intolerant to interferon alfa therapies with or without ribavirin. J Gastroenterol Hepatol. 2016; 31:1860–1867.

12. Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatmentnaive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomized, phase 3 trial. Lancet Infect Dis. 2015; 15:645–653.
13. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013; 368:1878–1887.

14. Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013; 368:1867–1877.

15. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014; 370:1993–2001.

16. Korean Association for the Study of the Liver. 2017 KASL clinical practice guidelines management of hepatitis C: treatment of chronic hepatitis C. Clin Mol Hepatol. 2018; 24:169–229.
17. Youssef SS, Seif SM. Association of liver inflammation with alpha-fetoprotein and treatment response in hepatitis C virus genotype 4 patients. J Exp Integr Med. 2014; 4:23–27.

18. Liu YR, Lin BB, Zeng DW, et al. Alpha-fetoprotein level as a biomarker of liver fibrosis status: a cross-sectional study of 619 consecutive patients with chronic hepatitis B. BMC Gastroenterology. 2014; 14:145.

19. Park SH, Park CK, Lee JW, et al. Efficacy and tolerability of peginterferon alpha plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Korea: a multicenter, retrospective observational study. Gut Liver. 2012; 6:98–106.

20. Heo NY, Lim YS, Lee HC, et al. High effectiveness of peginterferon alfa-2a plus ribavirin therapy in Korean patients with chronic hepatitis C in clinical practice. Clin Mol Hepatol. 2013; 19:60–69.

21. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002; 36(5 Suppl 1):S237–S244.

22. Kelleher B, Afdhal NH. Management of the side effects of peginterferon and ribavirin used for treatment of chronic hepatitis C virus infection. [Internet]. Waltham (MA): UpToDate Version 18.0;2014. [updated 2018 Jan 8; cited 2018 Jul 8]. Available from:. https://www.uptodate.com/contents/management-of-the-side-effects-of-peginterferon-and-ribavirin-used-for-treatmentof-chronic-hepatitis-c-virus-infection?search=Management%20of%20the%20side%20effects%20of%20peginterferon%20and%20ribavirin%20used%20for%20treatment%20of%20chronic%20hepatitis%20C%20virus%20infection&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1.
Go to : 
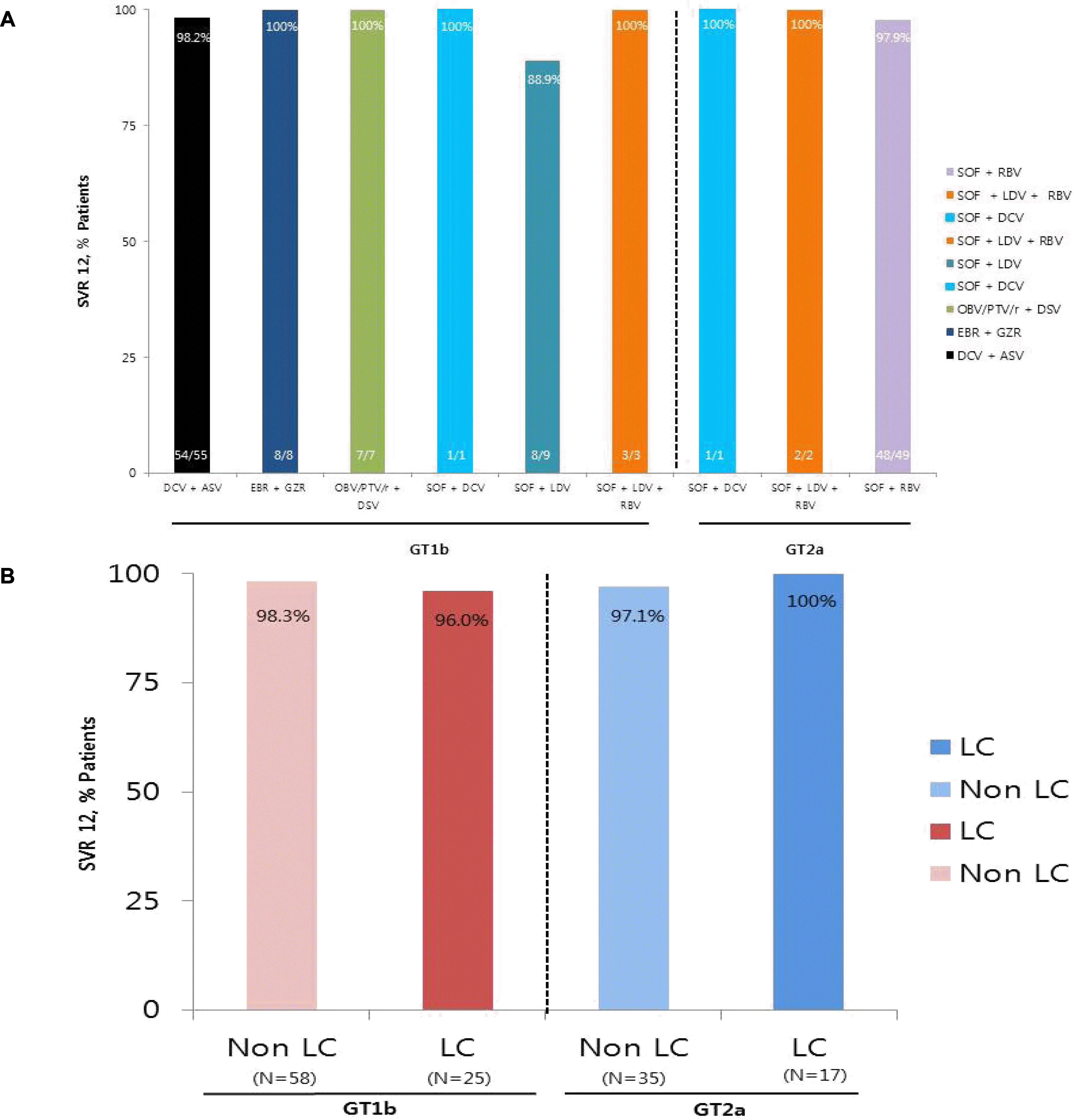 | Fig. 1.Virological response. (A) SVR 12 according to DAAs in each genotype. The result of DAA treatment for 1b and 2a showed high SVR 12 results without any significant difference in the treatment regimens. (B) SVR 12 according to the presence of cirrhosis in each genotype. SVR 12 rate showed similar results in both genotypes regardless of LC and non-LC. SVR, sustained virologic response; DCV, daclatasvir; ASV, asunaprevir; EBR, elbasvir; GZR, grazoprevir; OBV, ombitasvir; PTV, paritaprevir; r, ritonavir; DSV, dasabuvir; SOF, sofosbuvir; LDV, ledipasvir; RBV, ribavirin; GT, genotype; LC, liver cirrhosis; DAAs, direct-acting antivirals. |
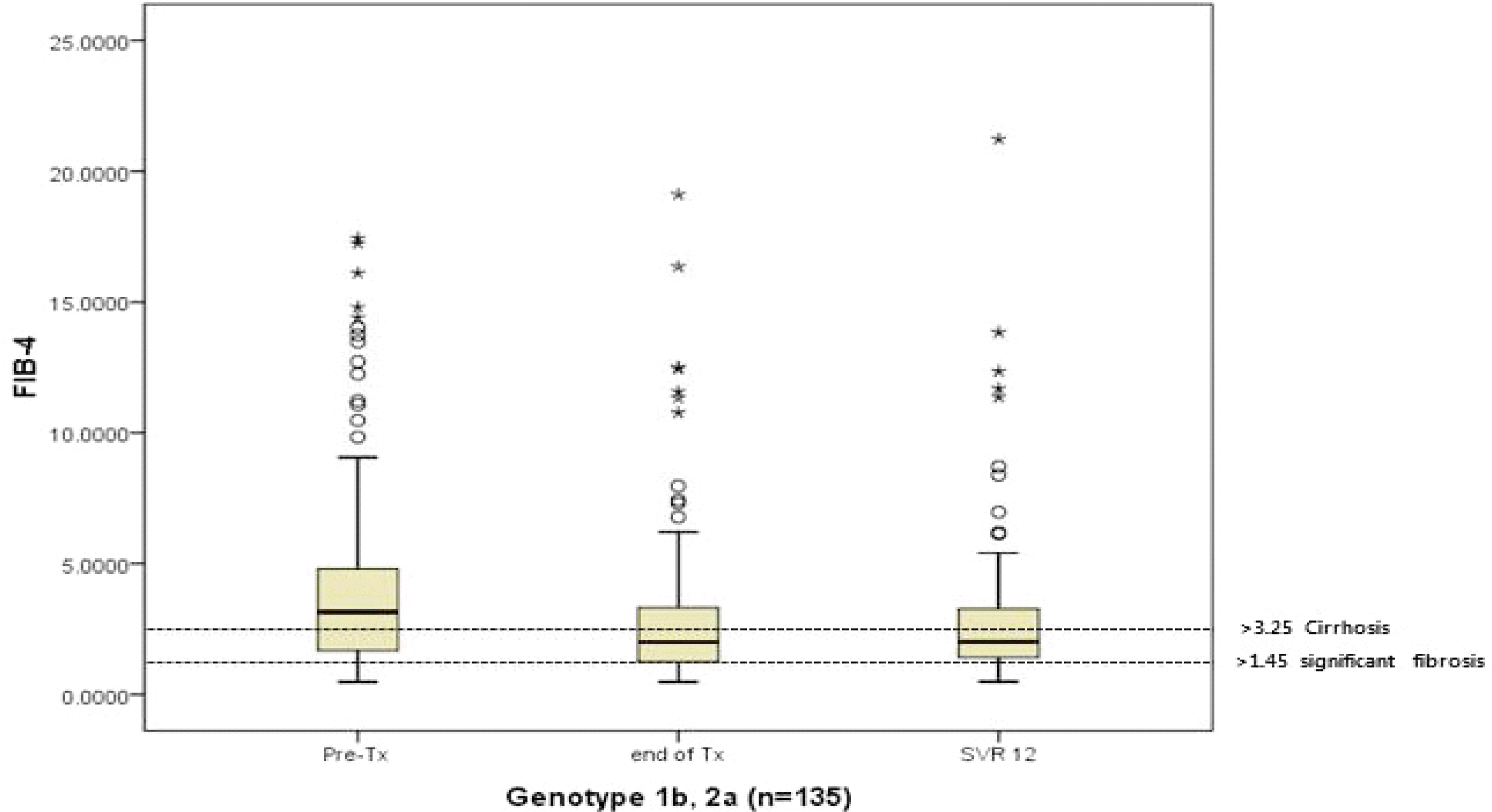 | Fig. 2.FIB-4 scores pre- and posttreatment with DAA in all patients (n=135). The median FIB-4 scores decreased from 4.15±3.72 at baseline to 2.84±2.78 at SVR 12 (p<0.001). FIB-4, fibrosis-4; Pre-Tx, pretreatment; SVR, sustained virologic response; DAA, direct-acting antiviral. |
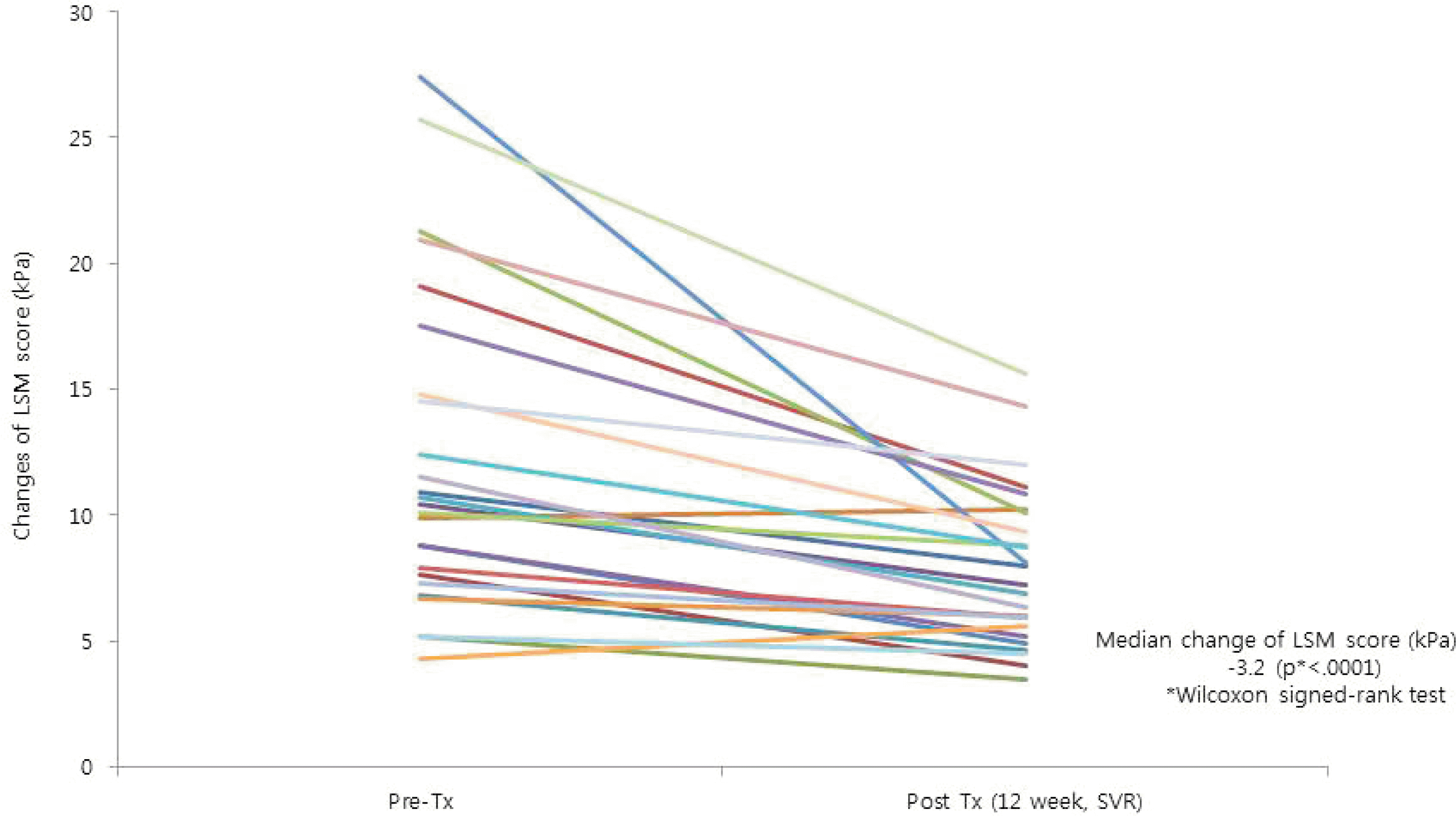 | Fig. 3.LSM score (n=25). Significant improvement in the LSM score was observed after successful DAA treatment. LSM, liver stiffness measurement; kPa, kilopascal; Pre-Tx, pretreatment; SVR, sustained virologic response; DAA, direct-acting antiviral. |
Table 1.
Baseline Clinical Characteristics of Patients
| Genotype 1b | Genotype 2a | |
|---|---|---|
| (n=83) | (n=52) | |
| Age (years) | 57.8±13.4 | 60.2±9.5 |
| Male | 44 (53.0) | 20 (38.5) |
| BMI (kg/m2) | 24.3±3.3 | 22.8±2.8 |
| HCV viral load (log10 IU/mL) | 6.6±6.7 | 6.4±6.7 |
| Prior HCV treatment | ||
| Treatment naive | 63 (75.9) | 44 (84.6) |
| Non-response | 14 (16.9) | 5 (9.6) |
| Relapse/breakthrough | 6 (7.2) | 3 (5.8) |
| Cirrhosis | 25 (30.1) | 17 (32.7) |
| Median ALT (ULN: 44 IU/L) | 70±71 | 66±49 |
| Median FIB-4 score | 3.89±3.53 | 4.58±4.02 |
| Median GFR (mL/min) | 70.6±19.4 | 74.4±13.6 |
| RASs | 11 (13.3) a | − |
| Treatment regimen | ||
| Daclatasvir+asunaprevir | 55 (66.3) | 0 (0.0) |
| Elbasvir+grazoprevir | 8 (9.6) | 0 (0.0) |
| OPr+dasabuvir | 7 (8.4) | 0 (0.0) |
| Sofosbuvir+ledipasvir | 9 (10.9) | 0 (0.0) |
| Sofosbuvir+daclatasvir | 1 (1.2) | 1 (1.9) |
| Sofosbuvir+ribavirin | 0 (0.0) | 49 (94.2) |
| Sofosbuvir+ledipasvir+ribavirin | 3 (3.6) | 2 (3.9) |
Table 2.
Treatment Response according to Genotype
| Genotype 1b (n=83) | Genotype 2a (n=52) | |
|---|---|---|
| HCV RNA <15 IU/mL, n/n (%) | ||
| On treatment | ||
| 4 week | 82/83 (98.8) | 50/52 (96.2) |
| 12 week a | 28/28 (100) | 52/52 (100) |
| 24 week | 55/55 (100) | − |
| Post treatment | ||
| 12 week (SVR) | 81/83 (97.6) | 51/52 (98.1) |
| Virological failure | ||
| On treatment | 0 | 0 |
| Relapse | 2 b | 1 c |
HCV, hepatitis C virus; RNA, ribonucleic acid; SVR, sustained virologic response; LC, liver cirrhosis; SOF, sofosbuvir; DCV, daclatasvir; ASV, asunaprevir; LDV, ledipasvir; RASs, resistance associated substitutions; RBV, ribavirin.
a One patient was LC-compensated treated with SOF+DCV for 16 weeks with non-responsive to previous DCV+ASV treatment
Table 3.
Adverse Events
| Genotype 1b (n=83) | Genotype 2a (n=52) | |
|---|---|---|
| Pts. with adverse events | 17 (20.5) | 34 (65.4) |
| Discontinuation of DAAs | 0 | 0 |
| Dyspepsia | 6 (7.2) | − |
| Insomnia | 3 (3.6) | 1 (1.9) |
| Fatigue | 3 (3.6) | − |
| Other minority a | 5 (6.0) | − |
| Anemia b | − | 31 (59.6) |
| Symptomatic bradycardia c | − | 1 (1.9) |
| Cough | − | 1 (1.9) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download