INTRODUCTION
METHODS
Animals
Induction of renal IRI
Renal function and histopathological studies
Immunohistochemistry
Quantification of serum IL-6
Quantitative real-time polymerase chain reaction (qRT-PCR)
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
Measurement of caspase-3 activity
Immunoblotting of Bcl-2 and Bax
Statistical analysis
RESULTS
Fimasartan attenuated the IRI-stimulated increase in serum BUN and Cr levels
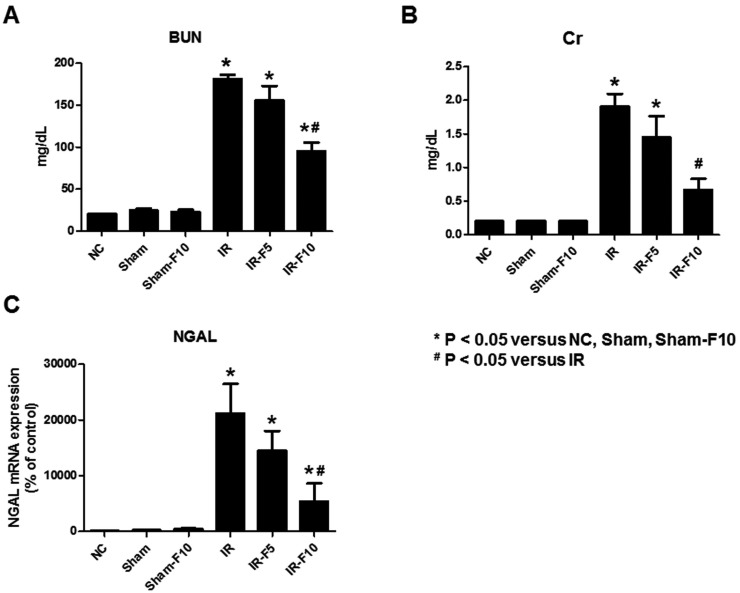 | Fig. 2Serum blood urea nitrogen (BUN), creatinine (Cr), and Neutrophil gelatinase-associated lipocalin (NGAL) levels in mice.Ischemia-reperfusion injury (IRI)-induces increases of (A) BUN, (B) Cr and (C) NGAL compared to control groups. Pretreatment with 10 mg/kg/day fimasartan attenuates the increase of BUN and Cr levels after IRI. The increased level of NGAL after IRI also significantly decreased after fimasartan treatment. Data are expressed as means±SEM (normal control, NC, n=5; sham operation, Sham, n=6; sham operation with 10 mg/kg/day fimasartan, Sham-F10, n=6; IR, n=8; IR with 5 mg/kg/day fimasartan, IR-F5, n=8; IR with 10 mg/kg/day fimasartan, IR-F10, n=8).
|
Fimasartan protected against IRI-induced renal injury
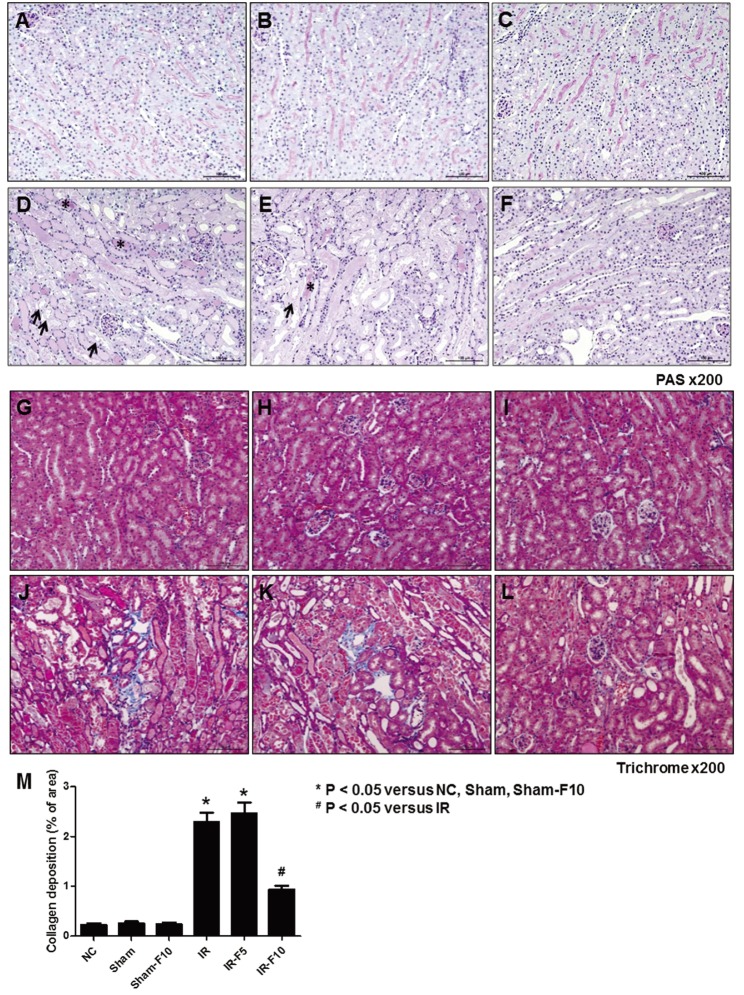 | Fig. 3Periodic acid-Schiff (PAS) and Masson's trichrome staining after ischemia-reperfusion injury (IRI).(A and G) Normal control (NC). (B and H) Sham operation (Sham). (C and I) Sham operation with 10 mg/kg/day fimasartan (Sham-F10). (D and J) IRI (IR). (E and K) IRI with 5 mg/kg/day fimasartan (IR-F5). (F and L) IRI with 10 mg/kg/day fimasartan (IR-F10). (M) Semi-quantitative assessment of the renal fibrosis area. Data are expressed as means±SEM. Tubular damage, loss of the brush border, and cast formation was significantly increased in the IR compared to the NC, Sham, and Sham-F10 groups, whereas the damages were decreased in the fimasartan-treated groups. Arrows indicate injured tubules and loss of brush border, and asterisks point to cast formation (NC, n=5; Sham, n=6; Sham-F10, n=6; IR, n=8; IR-F5, n=8; IR-F10, n=8).
|
Fimasartan attenuated the increased expression of pro-inflammatory cytokines following renal IRI
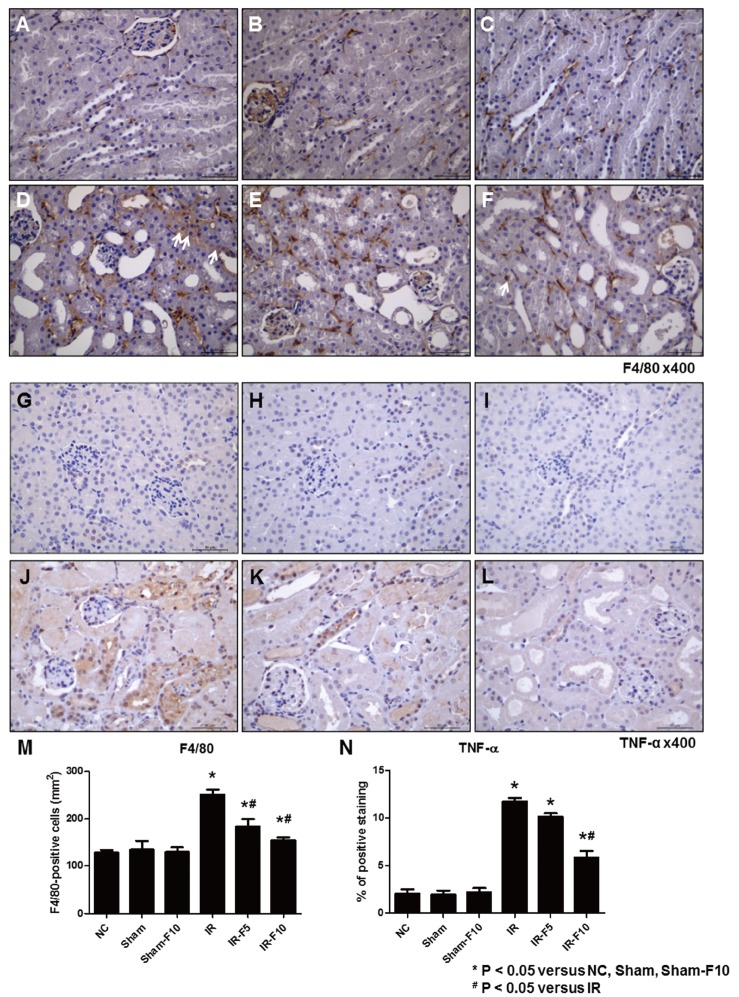 | Fig. 4Immunohistochemistry staining after ischemia-reperfusion injury (IRI).(A and G) Normal control (NC). (B and H) Sham operation (Sham). (C and I) Sham operation with 10 mg/kg/day fimasartan (Sham-F10). (D and J) IRI (IR). (E and K) IRI with 5 mg/kg/day fimasartan (IR-F5). (F and L) IRI with 10 mg/kg/day fimasartan (IR-F10). Immunohistochemistry shows increased labeling intensity of F4/80 and tumor necrosis factor (TNF)-α at the interstitial area after IRI (D and J) compared to NC (A and G), Sham (B and H), and Sham-F10 (C and I). Fimasartan treatment reverses the changes in labeling intensities of F4/80 and TNF-α in the IRI groups (F and L). (M and N) Semi-quantitative assessment of F4/80-positive cells and TNF-α-positive areas. Arrows indicate F4/80 positive cells. Data are expressed as means±SEM. (NC, n=5; Sham, n=6; Sham-F10, n=6; IR, n=8; IR with 5 mg/kg/day fimasartan, IR-F5, n=8; IR with 5 mg/kg/day fimasartan, IR-F10, n=8).
|
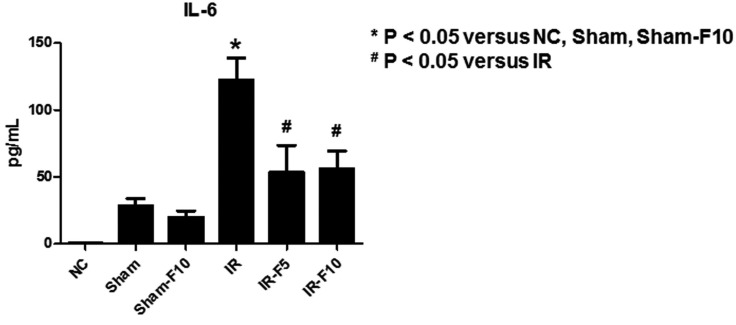 | Fig. 5Enzyme-linked immunosorbent assay results of serum interleukin (IL)-6 levels.IL-6 is significantly increased after renal ischemia-reperfusion injury (IRI) compared to the control groups. Pretreatment with fimasartan (5 mg/kg/day, IR-F5; 10 mg/kg/day, IR-F10) before IRI attenuates the change in IL-6. Data are expressed as means±SEM (normal control. NC, n=5; sham operation, Sham, n=6; sham operation with 10 mg/kg/day fimasartan, Sham-F10, n=6; IR, n=8; IR-F5, n=8; IR-F10, n=8).
|
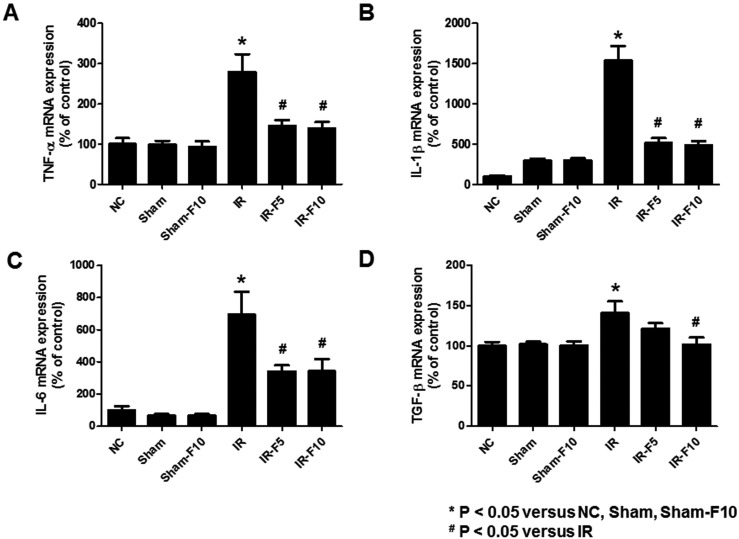 | Fig. 6mRNA levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and transforming growth factor (TGF)-β in mouse kidney tissue.Quantitative real-time polymerase chain reaction analyses revealed significant increases in the expression of (A) TNF-α, (B) IL-1β, (C) IL-6, and (D) TGF-β mRNA in the ischemia-reperfusion (IR) compared to control groups. Fimasartan treatment (5 mg/kg/day, IR-F5; 10 mg/kg/day, IR-F10) significantly inhibits the upregulation of TNF-α, IL-1β, IL-6, and TGF-β levels compared to the IR group. Data are expressed as means±SEM (normal control, NC, n=5; sham operation, Sham, n=6; sham operation with 10 mg/kg/day fimasartan, Sham-F10, n=6; IR, n=8; IR-F5, n=8; IR-F10, n=8).
|
Fimasartan attenuated apoptosis in renal IRI
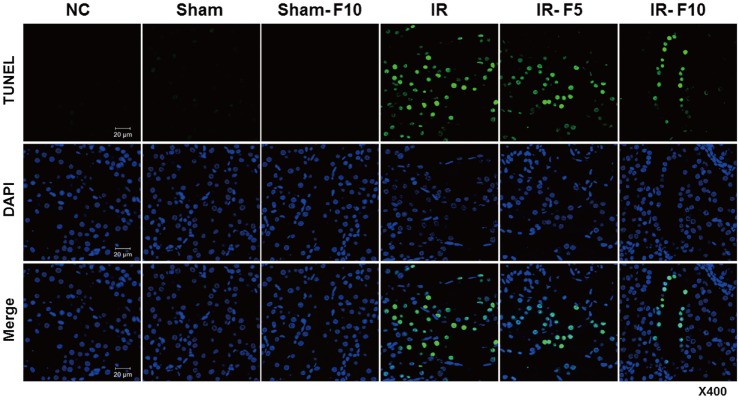 | Fig. 7Apoptosis of renal tubular epithelial cells.The TUNEL assay reveals that apoptosis induced in the ischemia-reperfusion (IR) group is significantly increased compared to that in the control groups. Apoptosis of renal tubule cells is decreased in the fimasartan-pretreated groups (5 mg/kg/day, IR-F5; 10 mg/kg/day, IR-F10) compared to the IR group.
|
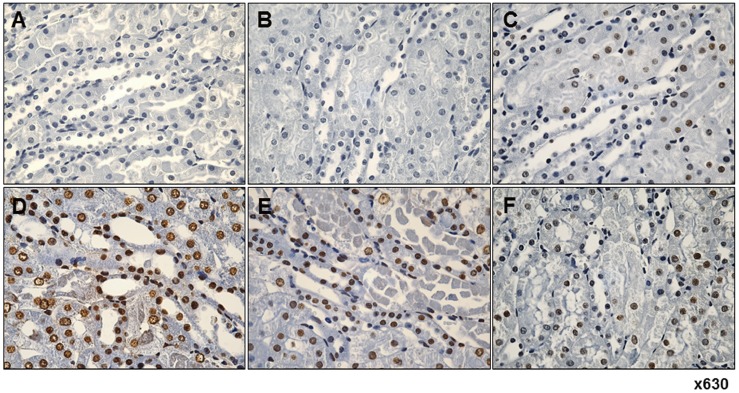 | Fig. 8Immunohistochemistry reveals fimasartan pretreatment suppresses ischemia-reperfusion injury (IRI)-induced apoptosis in tubular epithelial cells.(A) Normal control. (B) Sham operation. (C) Sham operation with 10 mg/kg/day fimasartan. (D) IRI. (E) IRI with 5 mg/kg/day fimasartan. (F) IRI with 10 mg/kg/day fimasartan. IR significantly increased the number of apoptotic cells compared with that in the sham group, which was significantly reduced by fimasartan. Arrows indicate TUNEL-positive cells in renal tubule.
|
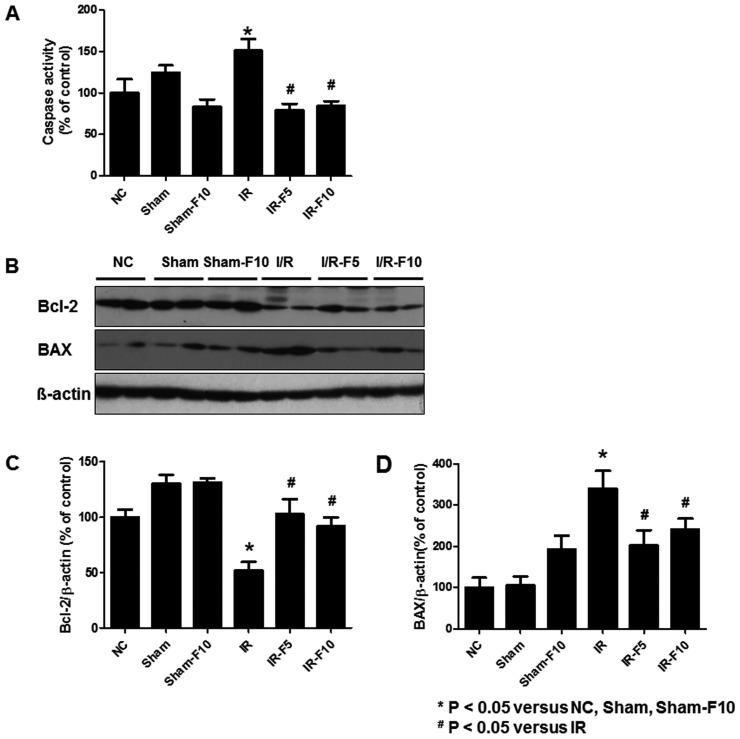 | Fig. 9Activity of caspase-3, and expression of Bcl-2 and Bax in renal tubular cells following ischemia-reperfusion injury (IRI).(A) Caspase-3 activity is significantly increased in the IR compared to the normal control (NC), sham operation (Sham), and sham operation with 10 mg/kg/day fimasartan (Sham-F10) groups. Fimasartan-treatment after IR (5 mg/kg/day fimasartan, IR-F5, and 10 mg/kg/day fimasartan, IR-F10) diminishes the increases of caspase-3. (B) Immunoblotting shows significantly decreased Bcl-2 and increased Bax levels in IR compared to NC, Sham, and Sham-F10. Quantitation of (C) Bcl-2 and (D) Bax levels. Pretreatment with fimasartan reverses the changes in Bcl-2 and Bax expression. Data are expressed as means±SEM.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download