INTRODUCTION
Infertility affects approximate 15% of couples and the male factors covers 20–50% of cases with the reduction of sperm quantity or/and quality [
1].
The medical treatment of infertility is divided into two main categories: specific and non-specific. The specific treatments are used for certain etiologies such as hypogonadotropic hypogonadism, male accessory gland infection, retrograde ejaculation, and positive anti-sperm antibody. These endocrine therapies include gonadotropins, androgens, anti-estrogens, and aromatase inhibitors [
2]. Non-specific treatment, also known as empirical medical treatment (EMT), divided into two categories, hormonal treatment [
3] and antioxidant supplementation [
4].
Fertility has been related with the central GHRH-growth hormone (GH)-IGFs endocrinal axis. Pituitary GH is involved in a wide array of reproductive functions in mammals such as sexual differentiation, pubertal maturation, gonad steroidogenesis, gametogenesis, and ovulation as well as pregnancy and lactation [
5]. Chubb [
6] reported that pituitary GH deficiency leads to a significant reduction of male sexual behavior or fertility. Bartke et al. [
7] reported that there were markedly reproductive deficits in GH receptor-knock-out or GH transgenic mice. Although most of the GH transgenic male mice are fertile, their fertility tends to be quantitatively reduced and plasma testosterone levels were not altered [
8]. Pituitary GH promotes sperm motility and longevity [
9]. Debeljuk et al. [
10] reported that the plasma testosterone and luteinizing hormone (LH) levels are normal in transgenic metallothionein-I/hGHRH mice, but the response to gonadotropin-releasing hormone (GnRH) was significantly less in the transgenic mice.
Some publications concerned the autocrine/paracrine GHRH and GH signals in the reproductive system. Berry et al. [
11] reported that extra-hypothalamic GHRH-like mRNA and immune-reactive peptide presented in rat testis and placenta, suggesting that testis and placenta are extra-hypothalamic sites of expression of GHRH gene. Martínez-Moreno et al. [
12] reported that GHRH co-localized with GH in the germinal epithelium and in interstitial zones within the chicken testes. GH affects the proliferation and differentiation functions of chicken reproductive tissues [
13].
These reports clearly reflected the relationship of the development of reproductive tract with pituitary GH. Although testicular GHRH, GHRH receptor, and GH signal molecules were discovered, their roles in fertility and the relationship with central GHRH-GH axis are not clearly known.
In our previous publication [
14],
Grin peptide (also known as 2F) showed the strongest and long-lasting
in vitro effect on rat GH release and similar species-specificity compared to natural hGHRH(1-44)NH
2. Up to now no publication about hGHRH agonist was reported in the pharmacodynamics of infertility. The treatment effect of
Grin on the infertility models of male hamsters were reported in the paper.
DISCUSSION
The effect of extra-hypothalamic GHRH might reflect the autocrine/paracrine GHRH receptor mediated functions.
Human hypothalamic GHRH has 93%, 71%, or 61% of identity with that from the Chinese hamster, rat, or mouse species (
Table 5). The difference of the C-terminal four amino acids in GHRH between Chinese hamster and human lead to less than 2% of the activity difference. Although GHRH species specificity is not too strict [
17], we still chose Chinese hamster (Cetulus griseus) as infertile animal models so that the species difference of GHRH molecule was overcome. Moreover Chinese hamster has an identity of 97, 97, or 67% in GH with mouse, rat, or human, so rat GH ELISA kit is easy to detect the hamster GH level. Chinese hamster is small like mouse, but its testis weight nearly covers 2% of body weight, so Chinese hamster is very suitable to be prepared as model of male infertility.
Cyclophosphamide is a regular clinic drug against some tumors. It is transformed into aldehyde phosphoramide by hepatic microsomal oxidase. Aldehyde phosphoramide is further degraded into amide nitrogen mustard and acrolein with alkylating function. By combining with loose DNA molecules in chromosome, DNA synthesis is inhibited in mitotic cells, i.e. spermatogonia and apermatocytes etc. But cyclophosphamide has weaker toxicity to quiescent cells, i.e. Leydig and Sertoli cells because the tightly wound chromatins in these cells are not combined easily by the alkylating agents. The infertility animal models with oligospermia or azoospermia are prepared to be based on the CPA toxicity to mitotic cells. Our preliminary research showed when CPA dose were no more than 200 mg/kg, Kunming mice kept 100% of survival rates, whereas the Chinese hamsters had 88.2% survival rates at 20 mg/kg.
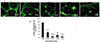
20 mg/kg-CPA-induced hamster models did not significantly alter in androgen level both in the 5-week modeling period and in the 5-week treatment period (data not shown), suggesting that the CPA dose did not impact the function of Leydig cells. In our preliminary research, when 10 hamsters (n=10) in each group were set (the minimal 6 hamsters were survived), the pregnancy rates (Grin 12.5–22.2% or hMG 22.2%) showed no significant difference compared to that (0%) of the single CPA group, suggesting that the animal models (n=10) was insufficient, so 17 hamsters in each group were used in the research. Because all the hamsters had been grouped before the animal experiment started, the final animals in each group were not uniformly distributed due to the different CPA tolerance. The final survivals of 13–16 hamsters suggest that the CPA dose was moderate to male hamsters. The higher androgen levels and more epithelial cells and clearance in the hMG group suggest that the CPA dose did not impact the functions of Sertoli and Leydig cells. In the TUNEL pictures, most of the TUNEL-positive cells are spermatogonia and spermatocytes, suggesting that the mitotic cells are more sensitive to CPA than the quiescent cells. In one word, the CPA dose only temporarily inhibited the proliferation of testicular mitotic cells to lead to infertility.
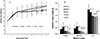
Before CPA ip, there was no significant difference in body weight between each group. After CPA injection, there was no statistical significance in body weight between experimental groups during the modeling period. In the treatment period,except that the seventh- and eighth-week hamsters in the M-Grin group or the eighth-week ones in the L-Grin group statistically showed less body weights than those in the single CPA or hMG group, there was not significant in body weight between the other time points in the experimental groups, which suggests that the parent hamster models may not impact the development of fetus and the growth of baby.
The more liver weight in the single CPA or hMG group suggests that CPA or hMG was metabolized in liver. Compared to the single CPA and hMGgroup, the less liver, testis, and body weights in the
Grin groups might result from the active fat degradation of GHRH-like peptide [
18], because we observed that there was less fat tissue distribution around testes and in abdomen in the
Grin groups. We will deeply search this in the future. The less liver weights also reflected that the
Grin hardly had stimulation to liver because
Grin had extremely low onset dose of 2 mg/kg (
i.m.) and maximal tolerated dose (MTD) of 4 g/kg (
i.v.). The H-
Grin more obviously protected testis weight from reduction than the M- or L-
Grin, because the H-
Grin promoted stronger proliferation of epithelial cells in the seminiferous tubules. Compared to the single CPA or
Grin groups, the more weights of hibateral testes in the hMG group suggest that hMG promotes growth of testis by releasing more androgen.
Our preliminary research showed when the hMG dose was less than 200 U/kg, the effect was less than that of the Grin groups in pregnancy rate. The pregnancy rate of H-, M-, L-Grin, or hMG group was 26.7, 30.8, 31.3, or 31.3%, respectively, showing significant differences compared to that (0%) of the single CPA group, but there was no difference between hMG and each Grin group. The hMG or H-Grin group had 12.5% or 13.3% of birth rates, suggesting that the H-Grin or hMG promotes early pregnancy. Because the hamster numbers (13–16) of each experimental group were not uniformly distributed, the calculated pregnancy rates of the H-, M-, L-Grin groups show a dose-inconsistent alteration. 2-8 mg/kg of Grin and 200 U/kg (46.2 mg/kg, 120 times equivalent to the clinical dose) of hMG showed similar therapeutic effect and different pathologic characteristics. The effect of Grin is at least 5.81 times stronger than that of hMG in a dose-effect relationship. In the single CPA group the loosely aligned tubules showed obvious intra-lumens, toxic swelling, and pathological vacuoles. The compactly arranged tubules in the Grin groups obviously showed the proliferation of germinal epithelial cells. All the results suggest that Grin promotes the proliferation and differentiation of primitive epithelial cells.
The ellipsoid seminiferous tubules in the Grin groups showed a dose-dependent enlargement, suggesting that the H-Grin strongly protects testis with reduction by promoting the proliferation of testicular cells, whereas hMG promotes growth of testis in efficacy.
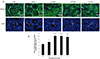
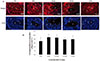
The results of GHRH receptor in the testis tissues confirm that there is obvious GHRH receptor distribution in primitive germinal cells. With the increasing of differential maturation, the expression of GHRH receptor gradually increased in the primitive germinal cells, suggesting that expression of GHRH receptor has a positive relationship with spermatogenesis. The more GHRH receptor expression in the Grin groups reflects the unique mechanism of Grin peptide.
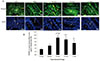
There was no significance in blood or testicular GH protein between the experimental groups, suggesting that pituitary or testicular GH did not follow the
Grin administration. The inconsistent result of GHRH receptor and GH suggests that the testicular GHRH receptor-mediated function may differ from GH in fertility. Some publications [
19] reported that GH directly provides a gonadotropin-dependent effect.
The distributions of GHRH receptor and GH proteins in the testicular tissue show similarity,
i.e. wide distribution in the testicular epithelial cells. But some characteristics were obvious. The testicular GHRH receptor presented a cell differentiation-dependent distribution, whereas the testicular GH uniformly distributes in all epithelial cells. Moreover the GH has stronger expression than the GHRH receptor. Although
Grin has the significant
in vitro pituitary GH release [
14],
in vivo the
Grin doses did not induce pituitary or testicular GH release, suggesting that the fertility effects of
Grin on the models were not mediated by GH. Also, the insignificant alteration in serum and testicular GHs may reflect that
Grin had an insufficient dose which did not trigger pituitary GH secretion, or more affinity to injury testes instead of pituitary.
Although some publications reported the reduced fertilities or the reproductive abnormalities of GH transgenic male mice [
20], or the treatment with hGH produced controversial results [
212223], the treatment effect of
Grin on male infertility hamsters was significant. Our research answers the relationship of the testicular GHRH receptor with fertility: an appropriate GHRH receptor level in testis may be necessary for fertility, which may be that testis contains a subset of GHRH target cells that have the capacity to respond to multiple releasing hormones and support fertility like anterior pituitary [
24], because we discovered that testicular GH cells are more than testicular GHRH receptor cells, indicating that the subset of cells may be multifunctional. Or, GHRH molecule has multiple signaling pathways to produce diverse functions, such as Adenylatecyclase→cAMP→PKA [
25], the voltage-gated Ca
2+ channels→Ca
2+ influx/intracellular Ca
2+ mobilization→Ca
2+↑ [
26], an alternative RNA processing mechanism of GHRH receptor gene [
27], phospholipase C→inositol phosphate-dependent pathway [
28], and mitogen-activated protein kinase pathway [
29].
Grin will be deeply studied to answer the difference with GH treatment in the future.







 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download