Abstract
Endometriosis (EM) is one of the most common gynaecological disorder affecting women in their reproductive age. Mechanisms involved in the pathogenesis of EM remains poorly understood, however inflammatory responses have been reported to be significantly involved. The efficacy of 6-shogaol on proliferation of endometriotic lesions and inflammatory pathways in experimentally-induced EM model was explored in this study. EM was stimulated in Sprague-Dawley rats by implantation of autologous endometrium onto the peritoneum abdominal wall. Separate groups were treated with 6-shogaol (50, 100 or 150 mg/kg b.wt/day) via oral gavage for one month period. Gestrinone (GTN) group received GTN (0.5 mg/kg/day) as positive control. Five weeks after implantation, the spherical volume of ecto-uterine tissues was determined. Treatment with 6-shogaol significantly reduced the implant size. Histological analysis reported atrophy and regression of the lesions. 6-shogaol administration effectively down-regulated NF-κB signaling, VEGF and VEGFR-2 (Flk-1) expression in the endometriotic lesions. Excess production of IL-1β and IL-6 (pro-inflammatory cytokines), PGE2 and nitric oxide (NO) were reduced. Overall, the results of the study reveal the efficacy of 6-shogaol against endometriosis via effectively suppressing proliferation of the lesions and modulating angiogenesis and COX-2/NF-κB-mediated inflammatory cascades.
Endometriosis (EM) is categorized by the growth of functional endometrium tissue exterior to uterus, commonly around the pelvic peritoneum and ovaries, affecting 5–10% of women in their reproductive age [12]. EM is associated with pelvic pain, infertility, dysmenorrhea, and dyspareunia [3]. Present treatment for EM is through surgery and/or hormonal therapy. Hormone treatment involves administration of progestogens, oral contraceptives, and gonadotropin-releasing hormone agonists [4]. However, these therapies are associated with serious adverse effects. Also, lesions reappear in 30–50% women within 3–5 years after surgery [5]. Identification of effective novel additional strategies in the treatment of endometriosis is of immense clinical value.
Understanding the pathogenesis of EM is crucial in development of effective treatment approaches. The development of endometrial lesions involves recruitment of blood vessels via angiogenesis to guarantee supply of oxygen and nutrients [6]. VEGF (vascular endothelial growth factor) is one of the chief pro-angiogenic factor that is involved in endometriosis [7]. VEGF exerts its effects via attaching to its receptors - as VEGF-A and VEGFR-2. Highly proliferative endometriotic lesions exhibit increased expression of VEGFR-2 and VEGF-A in the stroma and in blood vessels respectively [8].
Oxidative stress and inflammatory responses have also been said to be associated with the pathogenesis of EM [910]. Proinflammatory mediators as cytokines and prostaglandins are found to promote EM [111213]. Nuclear factor-kappa B (NF-kB), one of the chief transcription factor of the inflammatory process modulates various processes such as apoptosis, cell proliferation, adhesion, invasion and also angiogenesis in several cell types [14]. These cellular processes are documented to be crucially involved in endometriosis [291415]. Endometriotic lesions expresses increased cyclooxygenase-2 (COX-2) levels than eutopic endometrium [16]. Also, NF-κB induces COX-2 expression in the endometrial stromal cells [171819]. Thus, compounds that inhibit angiogenesis and the COX-2/NF-κB signalling pathways could be potential candidates in the treatment of EM.
Zingiber officinale (Ginger), is widely used in Asian cuisines and also as medicine for various ailments. Gingerols and its derivatives found in the rhizomes of ginger possess numerous biologically active properties [202122]. Shogaols, are gingerols that are found primarily in dried ginger [2023]. 6-shogaol reportedly exhibits wide range of pharmacological activities as antioxidant [24], anticancer [2526], neuroprotective [27] and anti-inflammatory effects [2829]. In this study the effects of 6-shogaol on the major pathways in endometriosis was explored.
Eight to nine weeks old Sprague-Dawley (Female) rats weighing 180–220 g were procured from the laboratory study animal care center of WuHan University and were housed under controlled environmental conditions (12 h light/dark cycle; 22±1℃; 55–60% humidity). Animals had unrestricted access to food and water and were acclimatized to in-house conditions for a week preceding to experiment. Approval of the animal study experiments were obtained from the WuHan University Animal Care and Use Committee and strict NIH Guidelines for the Care and Use of Laboratory Animals were followed [30].
6-Shogaol (Sigma-Aldrich, St.Louis, MO, USA), Fetal bovine serum (FBS) (Thermoscientific, USA), RPMI1640 medium (Gibco, Grand Island, NY, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), polyvinylidenedifluoride (PVDF) membranes and enhanced chemiluminescence (ECL) kit (Invitrogen) were used in the study. ELISA kits for determining cytokines-TNF-α, IL-1β and IL-6 were purchased from Biolegend (San Diego, CA, USA). Buffers used in Western blotting analysis were procured from Beyotime Institute of Biotechnology (Beijing, China). Antibodies against VEGF, VEGF-A, VEGFR-2 and COX-2 were procured from Cell Signaling Technology (Danvers, MA, USA). Horseradish peroxidase-labelled IgG secondary antibodies, PGE2, TNF-α, NF-κB p65, IκBα, p-IκBα, p-IKKβ, IKKβ, p-IKKα, IKKα and β-actin were purchased from Santa Cruz Biotechnology (Texas, USA).
A total of 75 rats (n=15/group) were used in this study. The induction of experimental endometriosis was done as described by Vernon and Wilson [31]. Briefly, a 3 cm midline incision in the abdomen was made to expose the uterus after anesthetizing the rats (i/m of xylazine and ketamine) and one uterine horn was excised and was split longitudinally and sectioned (5×5 mm pieces). The sections were transplanted into the peritoneum and rats were allowed to recuperate. Following 5 weeks after implantation, the spherical volume of ecto-uterine tissues was measured. The animals were randomly grouped into 6 experimental groups. Separate groups of rats were given 6-shogaol (50, 100 or 150 mg/kg b.wt) orally via gavage everyday 24 h following implantation for 30 days. The gestrinone (GTN) group were given GTN (0.5 mg/kg/day) [32] as positive control. Control group animals were not implanted with ectopic tissues nor were treated with GTN or shogaol but, were administered equal volume of saline. Endometriosis control group were implanted with ecotpic tissues but not administered with GTN or shogaol but given saline as in control group.
After 5 weeks, the treated rats were allowed to fast over-night and were sacrificed by cervical dislocation under isoflurane anesthesia. The endometrial tissues were excised with care and the spherical volume was measured as V (mm3)=height×width×length×0.52 mm [33]. The tissues (n=6/group) were then treated in phosphate buffered saline (PBS) and formalin fixed and embedded in paraffin for immunohistochemical and histological analysis.
The spherical volume of the ectopic endometrial tissues was measured using Vernier calipe. The difference in the growth and the of the cells following treatment were determined as volume change (mm3)=volume of EMS control (mm3)-Treatment volume (mm3).
Paraffin-embedded tissues were sectioned into 4 µm thickness and were hematoxylin and eosin (HE) stained and were observed under light microscope (Leica microsystems, Germany) at 200×magnification and analysed.
The expression of VEGF, VEGFR-2 (Flk-1), and COX-2 were assessed by immunohistochemistry. The sections were incubated with specific antibodies overnight (4℃). Following incubation for 40 min with secondary antibodies, the tissues were washed thrice with PBS and were treated with avidin-biotinylated peroxidase complex (ThermoFischer Scientific) for 40 min followed by diaminobenzidine (ThermoFischer Scientific). The tissue sections were then observed and quantified for positive cells with image processing and analysis software (NIS-Elements BR, Nikon Corporation, Japan).
Peritoneal fluid was collected from animals (n=6) after sacrifice by rinsing the abdominal cavity with PBS. The fluid was immediately centrifuged for 10 min (1,500 rpm) and supernatant was collected and stored at −70℃ until use.
Levels of IL-6, IL-1β (Biolegend, San Diego, CA, USA) and PGE2 (Cayman Chemical, Ann Arbor, MI, USA) were determined as per manufacturer's instructions. The levels were analysed using SpectraMax 190 automatic plate reader and were further analysed using SoftMax pro software (Molecular Devices, Sunnyvale CA, USA).
Nitric oxide levels in the peritoneal fluid was assessed using nitrate/nitrite kit (Cayman chemical, Ann Arbor, MI, USA). Nitrate reductase aids in converting nitrate to nitrite and by addition of Griess reagent nitrite produces a deep purple azo compound. At 540 nm, the compound was measured using ELISA reader. The absorbance of the azo compound accurately reflects nitrite concentration.
Using RIPA lysis buffer [50 mM Tris-HCl (pH 7.6), 1% NP-40; sodium deoxycholate (0.5%), SDS (0.1%), PMSF, Aprotinin (1 mg/L), Leupeptin (1 mg/L)] the endometrial tissue sections were homogenized on ice and centrifuged. Total protein concentration in the supernatant was determined using BCA assay kit (BioRad, USA). For NF-κB (p65) expression analysis both in nuclear and cytosolic fractions, aliquot of the tissue homogenate was separated into fractions as per manufacturer's protocol using an NE-PER nuclear and cytoplasmic extraction reagents kit (Pierce Biotechnology, Rockford, IL, USA). Protein concentration of equivalent volumes (50 µg) from each group were electrophoretically separated on SDS-PAGE (10%). The bands were transferred onto PVDF membrane (Invitrogen). The PVDF membrane was then blocked with TBST buffer (20 mM Tris -pH7.6; 137 mM NaCl; 0.1% Tween 20) that contained 5% non-fat milk at 37℃ for 60 min and were incubated overnight with respective primary antibody (1:1,000) at 4℃. The PVDF membranes were washed with TBST thrice and were incubated at 37℃ with secondary HRP-labelled antibodies (1:2,000) for 60 min. Positive bands were analyzed by chemiluminescence method (Millipore, USA) and analyzed by ChemiDoc XRS imaging system (Bio-Rad, USA). Using internal control (β-actin), the concentration of test proteins was normalized.
RT-PCR analysis was done to explore the influence of 6-shogaol on the levels of VEGF and Flk-1 m-RNA by TaqMan RT-PCR. The endometrial tissue total RNA was extracted as per manufacturer's protocol using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). Total isolated RNA (2 µg) was used as a template for synthesising cDNA and by using the SuperScript II reverse transcriptase kit (Invitrogen) the first strand was synthesized. With SYBR green fluorescence PCR was carried out using 7300 Real-Time PCR System (Applied Biosystems). The following primers were used for amplification - Flk-1-sense: 5′-GCACTGAATTATGGGAGA-3′, antisense-5′-ATGTGATTTTCTTCTTGATG-3′; VEGF-sense: 5′-ACCATGAACTTTCTGCTC-3′, antisense-5′-GGACGGCTTGAAGATATA-3′; GAPDH-sense: 5′-CACCACCATGGAGAAGGC-3′, antisense: 5′-CCATCCACAGTCTTCTGA-3′. The PCR products were then separated on agarose gel electrophoresis (2%). The bands were stained with ethidium bromide (0.05%) and the intensities were analyzed by Bio-Gel imagery apparatus (Bio-Rad, USA).
Obtained experimental data were statistically analyzed using SPSS software (version 21.0) (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) was performed for comparing multiple groups followed by Duncan's Multiple Range Test (DMRT) with values at p<0.05 as statistically significant.
Effect of 6-shogaol on the development of ectopic endometrial tissues were mainly evaluated by the volume change. In the present study volume of the ectopic endometrial tissues of in EMS control group increased significantly (p<0.05) as compared against standard control group. The spherical volume of ectopic tissues was observed to be reduced in rats treated with 6-shogaol (Table 1). GTN exposed animals also displayed decline in the volume as compared against EMS control. Further, the histopathological findings revealed marked atrophy and regressions of endometriotic lesions following 6-shogaol treatment like GTN (Fig. 1).
Angiogenesis is well documented as a main process in endometriosis pathogenesis. VEGF and Flk-1 expressions in the endometrial tissues were assessed by immunostaining. The immunological responses were found to be intense in endometriosis control group animals rather in eutopic endometrium of control group animals. Also, mRNA and protein levels of VEGF and Flk-1 as assessed by RT-PCR and western blotting analysis revealed similar observations (Figs. 2 and 3). The results suggest markedly enhanced (p<0.05) angiogenesis process in endometriosis. Further, GTN noticeably decreased VEGF and its receptor Flk-1. It was observed that 6-shogaol treatment for 30 days down-regulated VEGF and Flk-1 expression in a dose-dependent manner indicating inhibition of angiogenesis.
Inflammatory responses are known to be involved in progression of endometriosis. In this study the expression of proteins of the NF-κB pathway, and PGE2 and COX-2 were assessed. Levels of COX-2 expression in immunohistochemical staining and the intensity protein expression as determined by WB (Fig. 4a) were markedly enhanced. NF-κB signalling pathway was noticed to be significantly activated as reflected by elevated expression of NF-κB (p65) in nuclear fraction with considerable (p<0.05) decline in NF-κB (p65) cytosol fraction. Also, up-regulated expressions of PGE2, TNF-α, p-IKKα, p-IκBα and p-IKKβ were noticed (Figs. 4b and c) in endometriotic lesions. Administration of 6-shogaol substantially down-regulated NF-κB (p65) levels in nuclear fraction with substantial (p<0.05) decline in the levels of phosphorylated forms of major kinases of the pathway-IKKα, IKKβ, and IκBα indicating inhibition of the NF-κB pathway. The expressions of PGE2 and TNF-α were also noticeably suppressed on shogaol treatment. Shogaol at 150 mg/kg dose was found to be more effective in down-regulating expressions as compared to lower doses of 50 and 100 mg.
In line with protein expression, the levels of PGE2, IL-1β, IL-6, NO and inflammatory mediators were strikingly raised in the peritoneal fluid of endometriosis induced animals as compared with normal control group (Figs. 5a–c). GTN treatment bring about decline in the levels of PGE2, IL-1β and IL-6. 6-shogaol reduced the levels in a dose-dependent manner. The observations indicate significant ant-inflammatory potential of 6-shogaol.
Animal models of endometriosis are extensively employed for understanding the pathology underlying the progression of the disease and for evaluation of effective novel compounds for therapy. The ectopic auto-transplantation of uterine tissues is widely recognized method for inducing endometriosis in study rats [3134]. In this present work, effects of administration of 6-shogaol on growth of implanted endometriotic tissues and on the major pathways of inflammatory process in rats following induction of endometriosis was assessed. 6-Shogaol treatment for 30 days had resulted in declined growth of the endometriotic tissue implants as revealed by the decrease in the volume of the lesions and the histopathological findings indicated significant decrease in atrophy and lesion regression. The observations suggest the efficacy of 6-shogaol.
Inflammatory processes have been crucially involved in endometriosis. Constitutive activation of NF-κB signalling has been noticed in endometriotic lesions and in pelvic macrophages [3536]. NF-κB is found to be a proinflammatory, angiogenic, and a tissue-remodelling factor in endometrial stromal cell (ESC). Activation of the NF-kB pathway was observed in ESC cultures [3738]. COX-2 and MMPs are documented to be up-regulated by NF-κB [3940]. Studies illustrated an NF-kB-dependent expression of inflammatory mediators-COX-2, IL-8, and macrophagemigration inhibitory factor (MIF) in TNF-and IL-1β stimulated ESCs [41424344]. Under normal conditions NF-κB exists in an inactive state found bound to inhibitor proteins (IκB family of proteins) in the cytosol. Upon activation, NF-κBp65 migrates to the nucleus, eventually leading to the expression and release of several pro-inflammatory cytokines [454647]. Peritoneal macrophages exhibiting NF-kB activation has been reported in women with endometriosis [1936].
Expression of angiogenic factors including-COX-2, IL-1β, IL-6, TNF-α, iNOS, pro-inflammatory cytokines and VEGF secreted by activated peritoneal macrophages is known to be critically involved in pathogenesis of endometriosis are regulated by NF-κB [48495051525354]. In this study, activation of NF-κB signalling following induction of endometriosis was observed by increased levels of p65NF-κB in the nuclear fraction and increased phosphorylation of the major regulatory kinases of the pathway. The observed increase in levels of IL-6, IL-1β, COX-2, PGE2 and TNF-α following induction of endometriosis illustrate significant NF-κB activation and elevated inflammation. NF-κB mediated release of inflammatory cytokines consequently promote cell survival and induce the more inflammatory reactions in the lesions [19].
Many of the pro-inflammatory cytokines released by activated macrophages exert angiogenic activities [55]. VEGF is regarded to be a major pro-angiogenic factor in endometriosis [7]. In the study significantly, increased expression of Flk-1 and VEGF were observed in endometriotic lesions. Previous studies have also reported raised VEGF levels in the lesions and peritoneal fluid in endometriosis [56]. Increased Flk-1 expressions were reported by Wang et al. [57]. 6-Shogaol distinctly down-regulated the VEGF and Flk-1 expression both at mRNA and protein levels illustrating inhibition of angiogenesis. This reduced expression could have indirectly aided in reduction in viability of the ectoendometriotic tissues. Further, IL-1β, IL-6 and TNF-α induce VEGF expressions [3454]. Celik et al. [58] reported a significant reduction in microvessel density in a rodent endometriosis model by hindering the NF-κB pathway.
Here, raised levels of cytokines would have in part resulted in elevated VEGF levels and increased cell growth and survival. Studies have demonstrated that the decrease in IL-1β, down-regulates the COX-2 levels in ectopic endometria and inhibits PGE2 expressions that eventually can result in the decrease expressions of estrogen [59]. Also, PGE2 has been reported to induce VEGF expressions thereby contributing to angiogenesis in endometriosis [116061]. The results observed were in line with earlier reports [345462] suggesting the significance of the microenvironment of inflammatory mediators and angiogenic factors in progression of endometriosis. Inhibition of inflammatory responses via down-regulating NF-κB signalling and angiogenesis are critical in therapy of endometriosis.
Several studies have suggested that reduction in proliferation of endometriotic lesions involves the use of hormone therapies, anti-inflammatory drugs as COX-2 inhibitors and TNF-α inhibitors, inhibitors of MMPs, and anti-angiogenic agents [636465]. Here in the present study, 6-shogaol treatment significantly reduced levels of cytokines, PGE2, VEGF, COX-2 and NO that strikingly demonstrates the anti-inflammatory effects of shogaol. The results illustrate efficacy of 6-shogaol against progression of endometriosis via down-regulating growth, VEGF-mediated angiogenesis and NF-κB-mediated inflammation. Ergenoğlu et al. [66] demonstrated reduction in implant size and histological changes in the endometrial lesions following treatment with resveratrol. Administration of curcumin was found to decrease the ectopic endometrial glands [67]. These reports indicate the use of phytomedicines in endometriosis and, also support the results of the present investigation, thus making 6-shogaol a potential candidate drug that could be investigated more in therapy of endometriosis.
Figures and Tables
Fig. 1
Histopathological findings of endometriotic lesions following 6-shogaol treatment.
6-shogaol effectively caused regression of the lesions. (a) Representative photomicrograph presenting endometrotic lesions of EMS control group and (b) representative photomicrograph presenting regression and atrophy of lesions following 6-shogaol treatment at 150 mg/kg.
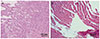
Fig. 2
6-Shogaol regulates the expression of VEGF and Flk-1.
(a) 6-Shogaol markedly down-regulates the expressions of VEGF and Flk-1 mRNA levels. (b) Relative mRNA expression levels. Values are represented as mean±SD, n=6. p<0.05 as determined by one-way ANOVA followed by DMRT analysis. *Represents p<0.05 vs. control; #Represents p<0.05 vs. EMS control. a–dRepresents mean values from different experimental groups that differ from each other at p<0.05.
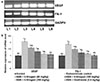
Fig. 3
Effect of 6-shogaol on VEGF and Flk-1 and COX-2 protein expressions.
6-Shogaol markedly regulates the expressions of VEGF and Flk-1 and COX-2 proteins (a). Representative immunoblot (b). Relative expressions of proteins. Values are represented as mean±SD, n=6. p<0.05 as determined by one-way ANOVA followed by DMRT analysis. Relative expressions of proteins from different experimental groups with control expressions set at 100%. *Represents p<0.05 vs. control; #Represents p<0.05 vs. EMS control. a–dRepresents mean values from different experimental groups that differ from each other at p<0.05.
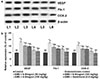
Fig. 4
6-Shogaol down-regulated NF-κB signaling.
6-Shogaol significantly inhibited activation and regulated the inhibitor kinases of NF-κB signaling (a). Representative Immunoblot exhibiting the influence of 6-shogaol on the expressions of proteins (b and c). Relative expression of proteins. Values are represented as mean±SD, n=6. p<0.05 as determined by one-way ANOVA followed by DMRT analysis. Relative expressions of proteins from different experimental groups with control expressions set at 100%. *Represents p<0.05 vs. control; #Represents p<0.05 vs. EMS control. a–eRe presents mean values from different experimental groups that differ from each other at p<0.05.
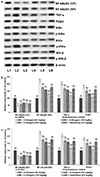
Fig. 5
Effect of 6-Shogaol on inflammatory cytokines.
6-Shogaol reduced the levels of inflammatory cytokines (a) PGE2 (b) and NO (c). Values are represented as mean±SD, n=6. p<0.05 as determined by one-way ANOVA followed by DMRT analysis. Relative expressions of proteins from different experimental groups with control expressions set at 100%. *Represents p<0.05 vs. control; #Represents p<0.05 vs. EMS control. a–fRepresents mean values from different experimental groups that differ from each other at p<0.05.

Notes
References
1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997; 24:235–258.

2. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005; 30:43–52.
3. Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E. ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005; 20:2698–2704.

4. Ruhland B, Agic A, Krampe J, Diedrich K, Hornung D. Innovations in conservative endometriosis treatment: an updated review. Minerva Ginecol. 2011; 63:247–259.
5. Guo SW, Olive DL. Two unsuccessful clinical trials on endometriosis and a few lessons learned. Gynecol Obstet Invest. 2007; 64:24–35.

6. Groothuis PG, Nap AW, Winterhager E, Grümmer R. Vascular development in endometriosis. Angiogenesis. 2005; 8:147–156.

7. Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci. 2002; 955:89–100. discussion 118, 396-406.

8. Bourlev V, Volkov N, Pavlovitch S, Lets N, Larsson A, Olovsson M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction. 2006; 132:501–509.

9. Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002; 77:861–870.

10. Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci. 2002; 955:183–198. discussion 19-200, 396-406.

11. Laschke MW, Elitzsch A, Scheuer C, Vollmar B, Menger MD. Selective cyclo-oxygenase-2 inhibition induces regression of autologous endometrial grafts by down-regulation of vascular endothelial growth factor-mediated angiogenesis and stimulation of caspase-3-dependent apoptosis. Fertil Steril. 2007; 87:163–171.

12. Tariverdian N, Theoharides TC, Siedentopf F, Gutiérrez G, Jeschke U, Rabinovich GA, Blois SM, Arck PC. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007; 29:193–210.

13. Wu MH, Shoji Y, Chuang PC, Tsai SJ. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert Rev Mol Med. 2007; 9:1–20.

14. Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007; 8:49–62.
16. Chishima F, Hayakawa S, Sugita K, Kinukawa N, Aleemuzzaman S, Nemoto N, Yamamoto T, Honda M. Increased expression of cyclooxygenase-2 in local lesions of endometriosis patients. Am J Reprod Immunol. 2002; 48:50–56.

17. Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006; 119:229–240.

18. Kim YA, Kim JY, Kim MR, Hwang KJ, Chang DY, Jeon MK. Tumor necrosis factor-alpha-induced cyclooxygenase-2 overexpression in eutopic endometrium of women with endometriosis by stromal cell culture through nuclear factor-kappaB activation. J Reprod Med. 2009; 54:625–630.
19. González-Ramos R, Van Langendonckt A, Defrère S, Lousse JC, Colette S, Devoto L, Donnez J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil Steril. 2010; 94:1985–1994.

20. Suekawa M, Ishige A, Yuasa K, Sudo K, Aburada M, Hosoya E. Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J Pharmacobiodyn. 1984; 7:836–848.
21. Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998; 129:139–144.

22. Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Kadomatsu K, Goto H. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Commun. 2007; 362:218–223.

23. Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008; 46:409–420.
24. Bak MJ, Ok S, Jun M, Jeong WS. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012; 17:8037–8055.

25. Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007; 45:683–690.

26. Ray A, Vasudevan S, Sengupta S. 6-Shogaol inhibits breast cancer cells and stem cell-like spheroids by modulation of notch signaling pathway and induction of autophagic cell death. PLoS One. 2015; 10:e0137614.

27. Seow SLS, Hong SL, Lee GS, Malek SNA, Sabaratnam V. 6-shogaol, a neuroactive compound of ginger (jahe gajah) induced neuritogenic activity via NGF responsive pathways in PC-12 cells. BMC Complement Altern Med. 2017; 17:334.

28. Park G, Oh DS, Lee MG, Lee CE, Kim YU. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol Appl Pharmacol. 2016; 310:51–59.

29. Han Q, Yuan Q, Meng X, Huo J, Bao Y, Xie G. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget. 2017; 8:42001–42006.
30. National Research Council. Committee for the update of the guide for the care and use of laboratory animals. Institute for Laboratory Animal Research. Division on Earth and Life Studies. Guide for the care and use of laboratory animals. 8th ed. Washington, D.C.: National Academy Press;2011.
31. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985; 44:684–694.
32. Tang Q, Shang F, Wang X, Yang Y, Chen G, Chen Y, Zhang J, Xu X. Combination use of ferulic acid, ligustrazine and tetrahydropalmatine inhibits the growth of ectopic endometrial tissue: a multi-target therapy for endometriosis rats. J Ethnopharmacol. 2014; 151:1218–1225.

33. Yildirim G, Attar R, Ozkan F, Kumbak B, Ficicioglu C, Yesildaglar N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: a preliminary study. Fertil Steril. 2010; 93:1787–1792.

34. Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004; 82:Suppl 3. 1008–1013.

35. González-Ramos R, Donnez J, Defrère S, Leclercq I, Squifflet J, Lousse JC, Van Langendonckt A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007; 13:503–509.

36. Lousse JC, Van Langendonckt A, González-Ramos R, Defrère S, Renkin E, Donnez J. Increased activation of nuclear factor-kappa B (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertil Steril. 2008; 90:217–220.
37. Tamura M, Sebastian S, Yang S, Gurates B, Ferrer K, Sasano H, Okamura K, Bulun SE. Up-regulation of cyclooxygenase-2 expression and prostaglandin synthesis in endometrial stromal cells by malignant endometrial epithelial cells. A paracrine effect mediated by prostaglandin E2 and nuclear factor-kappa B. J Biol Chem. 2002; 277:26208–26216.
38. Han S, Sidell N. RU486-induced growth inhibition of human endometrial cells involves the nuclear factor-kappa B signaling pathway. J Clin Endocrinol Metab. 2003; 88:713–719.
39. Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006; 210:171–186.

40. Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ. 1999; 10:353–367.
41. Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002; 87:3263–3273.
42. Cao WG, Morin M, Metz C, Maheux R, Akoum A. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor NFkappaB. Biol Reprod. 2005; 73:565–570.
43. Cao WG, Morin M, Sengers V, Metz C, Roger T, Maheux R, Akoum A. Tumour necrosis factor-alpha up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-kappaB. Hum Reprod. 2006; 21:421–428.
44. Chen SU, Lee H, Chang DY, Chou CH, Chang CY, Chao KH, Lin CW, Yang YS. Lysophosphatidic acid mediates interleukin-8 expression in human endometrial stromal cells through its receptor and nuclear factor-kappaB-dependent pathway: a possible role in angiogenesis of endometrium and placenta. Endocrinology. 2008; 149:5888–5896.
45. Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000; 18:621–663.
46. Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001; 2:675–680.

47. Boulanger D, Bureau F, Mélotte D, Mainil J, Lekeux P. Increased nuclear factor kappaB activity in milk cells of mastitis-affected cows. J Dairy Sci. 2003; 86:1259–1267.
48. Chilov D, Kukk E, Taira S, Jeltsch M, Kaukonen J, Palotie A, Joukov V, Alitalo K. Genomic organization of human and mouse genes for vascular endothelial growth factor C. J Biol Chem. 1997; 272:25176–25183.

49. McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000; 6:45–55.

50. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001; 76:1–10.

51. Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, Tsai SJ. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002; 8:1103–1110.

52. Wu MY, Chao KH, Yang JH, Lee TH, Yang YS, Ho HN. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Hum Reprod. 2003; 18:2668–2671.

53. Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J Cell Sci. 2003; 116:665–674.
54. Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006; 147:1278–1286.

55. Becker CM, D'Amato RJ. Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc Res. 2007; 74:121–130.

56. Machado DE, Berardo PT, Palmero CY, Nasciutti LE. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J Exp Clin Cancer Res. 2010; 29:4.

57. Wang HB, Lang JH, Leng JH, Zhu L, Liu ZF, Sun DW. Expression of vascular endothelial growth factor receptors in the ectopic and eutopic endometrium of women with endometriosis. Zhonghua Yi Xue Za Zhi. 2005; 85:1555–1559.
58. Celik O, Hascalik S, Elter K, Tagluk ME, Gurates B, Aydin NE. Combating endometriosis by blocking proteasome and nuclear factor-kappaB pathways. Hum Reprod. 2008; 23:2458–2465.
59. Ebert AD, Bartley J, David M. Aromatase inhibitors and cyclooxygenase-2 (COX-2) inhibitors in endometriosis: new questions--old answers? Eur J Obstet Gynecol Reprod Biol. 2005; 122:144–150.

60. Olivares C, Bilotas M, Buquet R, Borghi M, Sueldo C, Tesone M, Meresman G. Effects of a selective cyclooxygenase-2 inhibitor on endometrial epithelial cells from patients with endometriosis. Hum Reprod. 2008; 23:2701–2708.

61. Machado DE, Berardo PT, Landgraf RG, Fernandes PD, Palmero C, Alves LM, Abrao MS, Nasciutti LE. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis with an antiangiogenic effect in a rat model. Fertil Steril. 2010; 93:2674–2679.

62. Machado DE, Rodrigues-Baptista KC, Alessandra-Perini J, Soares de Moura R, Santos TA, Pereira KG, Marinho da Silva Y, Souza PJ, Nasciutti LE, Perini JA. Euterpe oleracea extract (Açaí) is a promising novel pharmacological therapeutic treatment for experimental endometriosis. PLoS One. 2016; 11:e0166059.

63. Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997; 99:2851–2857.

64. Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, Fried G, D'Hooghe TM. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006; 21:1856–1862.

65. Mihalyi A, Simsa P, Mutinda KC, Meuleman C, Mwenda JM, D'Hooghe TM. Emerging drugs in endometriosis. Expert Opin Emerg Drugs. 2006; 11:503–524.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download