Abstract
The effect of melatonin on juveniles with cardio fibrosis is poorly understood. We investigated whether HDACs participate in the anti-fibrotic processes regulated by melatonin during hypertrophic remodeling. Abdominal aortic constriction (AAC) was employed in juvenile rats resulting in pressure overload-induced ventricular hypertrophy and melatonin was subsequently decreased via continuous light exposure for 5 weeks after surgery. AAC rats displayed an increased cross-sectional area of myocardial fibers and significantly elevated collagen deposition compared to sham-operated rats, as measured by HE and Masson Trichrome staining. Continuous light exposure following surgery exacerbated the increase in the cross-sectional area of myocardial fibers. The expression of HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 genes were all significantly enhanced in AAC rats with light exposure relative to the other rats. Moreover, the protein level of TNF-α was also upregulated in the AAC light exposure groups when compared with the sham. However, Smad4 protein expression was unchanged in the juveniles' hearts. In contrast, beginning 5 weeks after the operation, the AAC rats were treated with melatonin (10 mg/kg, intraperitoneal injection every evening) or vehicle 4 weeks, and sham rats were given vehicle. The changes in the histological measures of cardio fibrosis and the gene expressions of HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 were attenuated by melatonin administration. The results reveal that melatonin plays a role in the development of cardio fibrosis and the expression of HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 in cardiomyocytes.
Heart failure is a leading cause of death in pediatric patients, yet fibrosis and its contribution to heart failure are still poorly understood. Investigative researches into the regulation of fibrosis and new therapeutic approaches to heart failure will be critical for the prevention and treatment of cardiac dysfunction in children.
Melatonin (N-acetyl-5-methoxytryptamine) is primarily synthesized in the pineal gland in humans, and its release peaks during the night [1]. Melatonin and its metabolites are potent radical scavengers and antioxidant agents [12]. Research into the molecular basis of melatonin's effects on the cardiovascular system is now rapidly evolving. In animal models of cardiac dysfunction, melatonin deficiency induced by continuous light exposure or pinealectomy results in hypertension [3]. On the other hand, melatonin treatment in hypertension patients helps to lower blood pressure [4].
Histone deacetylases (HDACs) remove acetyl groups from histone tails, and thus play a vital role in the regulation of gene expression [5]. There are four classes of HDACs: class I (1, 2, 3 and 8), class IIa (4, 5, 7 and 9), class IIb (6 and 10), and class IV (HDAC11), and all of these HDACs enzymes are zinc-dependent. The catalytic activity of Class III HDACs (SirT 1–7), in contrast, depend instead on nicotinamide adenine dinucleotide (NAD+) [6]. There is currently intense interest in the anti-fibrotic capacity of HDACs in combating pathologic cardiac fibrosis: HDAC inhibitors can reduce ventricle hypertension and fibrosis in various cardiovascular disease models [78]. Importantly, however, the molecular mechanisms underlying HDAC action remain poorly understood.
Recent work demonstrated that melatonin can prevent neonatal dexamethasone-induced hypertension through inhibition of histone deacetylases [9]. As a result, we hypothesized that melatonin may improve cardiac fibrosis in juvenile rats with overload pressure-induced heart failure.
Juvenile male Sprague Dawley rats (50–80 g) aged from 21 to 28 days were housed in an air-conditioned room with free access to food and water and were maintained on a 12:12 h light-dark cycle with independent ventilation, temperature, and humidity controls. All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and efforts were made to minimize suffering. The Ethics Committee of the Children's Hospital of Chongqing Medical University (Permit Number: SYXK2007-0016) approved all experiments. All animals (SPF grade) were purchased from the Animal Experiment Center of Chongqing Medical University.
A heart failure model was established by abdominal aortic constriction (AAC) according to previously described methods [10]. In brief, naive rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g). Through an abdominal incision, the abdominal aorta, located about 5 mm above the right renal vein, was ligated in parallel with a polished 23 G needle and a polyester suture (4-0). The needle was then gently extracted, resulting in a constricted abdominal aorta (0.6 mm in diameter) and the incision was sutured. Sham-operated rats underwent a similar surgical procedure but without the abdominal aorta ligation.
In the first part of investigation, the AAC-operated rats were randomly divided into two groups: one group was maintained under continuous 24 h light exposure—the AAC+Light group— while, the other was under normal light conditions with a 12:12 h light-dark cycle— the AAC group—for 5 weeks after surgery. The sham-operated rats—the Sham group—under normal light conditions.
In the second part of the study, beginning 5 weeks after surgery, rats with the AAC procedure were randomly assigned to receive either melatonin (10 mg/kg by intraperitoneal injection once every evening between 23:00 and 24:00; AAC+melatonin) [11] or vehicle (0.5% alcohol; AAC) for 4 weeks. The sham group received vehicle and served as the control group (sham).
Freshly isolated rat hearts from all experimental groups were fixed in 4% paraformaldehyde for at least 24 h. Heart tissues were then processed routinely for dehydration with 70–100% graded alcohol and embedded in blocks of paraffin wax. Serial sections of 4 µm thickness were cut and mounted on silanized slides, then, dried in an oven overnight at 60℃. Morphometric analysis of heart tissues from all experimental groups were conducted using hematoxylin and eosin (H–E) [12] staining, and collagen accumulation was assayed by Masson collagen staining [13]. The cross-sectional area of the myocardial fibers and the proportion of collagen deposition were quantified using Image-Pro Plus 6.0 Analyzer. All sections from each experimental group were examined by the same researcher.
Total RNA was extracted from the juvenile hearts using an RNA isolation kit (Bioteke Corporation, Beijing, China) and the RNA concentration was assessed spectrophotometrically at 260 nm. cDNA was synthesized using a reverse transcriptase kit (Takara, Japan). cDNA was then amplified by real time PCR in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Data were collected and analyzed using ABI Sequence Detector Software and normalized using GAPDH as the reference gene. mRNA expression was analyzed using the ΔCT method.
Total protein was extracted from cardiac tissue (KeyGEN BioTECH, China) and quantified using the BCA assay (BioTeke Biotechnology, China). Total protein (50 µg per lane) from the homogenate of isolated ventricular myocytes was separated by SDS-PAGE (Beyotime Biotechnology, China) and transferred to a PDVF membrane. The PVDF membrane was blocked with Trisbuffered saline plus Tween (0.05%) and 5% skim milk at room temperature for 1 h, then incubated with mouse anti-TNF-α (1:1000 dilution, abcam, USA), rabbit anti-Smad4 (1:1000 dilution, abcam, USA), or mouse anti-GAPDH (1:5000 dilution, Arigo, Taiwan) antibodies overnight at 4℃. The membranes were then washed with T-TBS solution and incubated with HRP-conjugated secondary antibody (1:5000 dilution, Lianke, China) for 1 h. The membranes were finally developed using a super ECL assay kit (KeyGEN BioTECH, China) and imaged with a G-BOX imaging system (Syngene, UK).
All statistical analyses were performed with SPSS software (version 19.0). Comparisons between the groups were performed using one-way ANOVA, and the LSD method was applied to estimate pairwise comparisons. Significance in statistical tests was set to p<0.05. Data are presented as the mean±standard deviation.
To evaluate whether the decreased melatonin induced via continuous light exposure could affect the development of cardio fibrosis in juveniles, we performed HE (Fig. 1A) and Masson's Trichrome staining (Fig. 1B) in heart tissues. As shown in Fig. 1C and 1D, the cross-sectional area of myocardial fibers (a measure of cardiomyocyte cell size and thus cardiac hypertrophy) and collagen disposition were significantly enhanced in the AAC group compared with those in the Sham group (all p<0.001). Interestingly, continuous light exposure exacerbated the increase in the cross-sectional area of myocardial fibers, but had no effect on collagen disposition, which was similar in the AAC+Light group and the AAC group.
To determine whether melatonin might have a therapeutic effect on cardio fibrosis, we administrated melatonin (10 mg/kg/day) or vehicle to AAC rats for 4 weeks, beginning five weeks after the surgery. As shown in Fig. 2, the increase in the cross-sectional area of myocardial fibers and deposition of interstitial collagen observed in vehicle-treated AAC heart was improved by melatonin treatment (all p<0.001).
HDACs participate in the progression of fibrosis diseases [14]. We therefore performed RT-PCR analysis to investigate whether HDACs gene expressions changes in the hearts of juvenile rats with cardiac dysfunction. As shown in Fig. 3, class I HDACs including HDAC1and HDAC2, and class IIb HDAC6 were significantly elevated in the AAC hearts than that in the sham hearts, and light exposure aggravated these changes (all p<0.001). Class I HDAC3 and class IIa HDAC4 were increased in the AAC+Light group compared to the other groups (all p<0.001). Melatonin administration, however, reduced the expression of these HDAC genes (Fig. 4; p<0.001, p=0.002, p<0.001, p=0.007, p<0.001, respectively).
Since dysregulation of TNF-α and Smad4 is associated with fibrosis in diverse organ systems, we performed western blot analysis to determine whether melatonin affected the expression of these proteins. As shown in Figs. 5A and 5B, the protein expression of TNF-α was increased in the AAC hearts relative to that in the sham hearts (p=0.031), and effect that was unchanged by melatonin treatment (p>0.05) (Figs. 6A and 6B). We also observed that the expression of Smad4 was the same in all groups (Figs. 5A, 5C, 6A and 6C) (all p>0.05).
The major finding in this study was that melatonin can prevent the cardio fibrosis normally caused by afterload pressure in juvenile rats. Decreasing melatonin levels by continuous light exposure dramatically increased HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 mRNA expression in failing hearts. The changes in HDAC expression were prevented by treatment with melatonin, suggesting that melatonin may play an inhibitory role in HDAC expression to guard against the development of cardio fibrosis in juveniles.
The over-afterload pressure induced by AAC resulted in cardio fibrosis and myocardiac remodeling, and reduced melatonin exacerbated these changes. These findings are in agreement with previous work showing that heart weights increased after pinealectomy in rats, indicating ventricle remodeling [15]. In this study, melatonin treatments from 5 to 9 weeks after AAC reverse these changes.
It is widely believed that HDACs are associated with epigenetic regulations of gene expressions and that HDAC inhibitors have direct effects on cardiac fibroblasts [16]. A recent study described how HDAC inhibitors work through Angiotensin II signaling to regulate cellular infiltration into the mouse heart [14]. Previous data also suggests that class I and class II HDACs play opposing roles in the regulation of hypertrophy. Class I HDACs are required for some hypertrophic responses and might normally work in the heart to repress antihypertrophic pathways, whereas class II HDACs may repress hypertrophy, though the experimental evidence in support of this hypothesis is lacking [17]. Lkhagva B and his colleagues measured the protein levels of HDACs (including HDAC1, HDAC2, HDAC3 HDAC8 in Class I, and HDAC4, HDAC6, HDAC9, HDAC10 in Class II) and found that the left ventricles of HF rats expressed significantly more HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 than the healthy left ventricles did [18]. Another study, performed with human heart samples, showed that HDAC4 represses pro-hypertrophic gene expression [19], while yet another study found that YY1 functions as an anti-hypertrophic factor by preventing HDAC5 nuclear export [20]. As a result, we chose to focus on these HDACs with reported ties to cardiac hypertrophy. While it has been found that HDAC7 is associated with gastric cancer, type 2 diabetes, and memory formation and that HDAC9 plays a role in cerebral ischemic stroke, lymphoma and acute coronary syndrome, there has been little research into the effect of HDAC7 or HDAC9 on cardiac hypertrophy. Our data demonstrate that afterload pressure-induced cardio fibrosis is associated with increased HDAC1, HDAC2 and HDAC6 gene expression, and that decreased melatonin via continuous light exposure exacerbates this effect. According to a previous study [21], transgenic overexpression of HDAC1 or HDAC3 in the heart did not induce cardiac hypertrophy or heart failure. The aberrant upregulation of HDAC1 and 3 occurred “after transition of heart failure”; in other words, HDAC1 and 3 were not responsible for inducing heart failure but rather secondary changes that resulted from the heart failure. Importantly, we also found that melatonin administration prevented the increase in HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 gene expression following AAC in juvenile heart, consistent with previous studies showing that melatonin plays a role as an HDAC inhibitor [922]. Nonetheless, the potential limitations of our study should be noted and we will consider looking at these additional HDACs in a future replication of this work.
TNF-α plays a fundamental role in the pathological changes seen in failing hearts, such as ventricular remodeling, cardio fibrosis and apoptosis [232425]. While our data demonstrate that protein expression of TNF-α was elevated in failing hearts, that the increase in TNF-α was not reversed by melatonin treatment. In another study, TGF-β and its downstream signaling protein Smad4 contributed to the pathology of heart failure [26], but the level of Smad4 was unchanged in our juvenile rats with cardiac dysfunction. The age of rats may response for the reason why the results were different than previous work has reported.
Many basic biological phenomena are related to circadian rhythms, such as body temperature, pulse, blood pressure, blood sugar, oxygen consumption, urination, basal metabolic rate, hormone secretion, wake-sleep. To exclude the circadian bias, these indicators of circadian rhythms should be measured among all the groups or balanced across groups. Light influences sleep indirectly through circadian pathway. Jessica W. Tsai and her colleagues assessed the role of melanopsin in mediating the effects of light on sleep and found that melanopsin contributes directly to the effects of light and darkness, and interact with the circadian and homeostatic drive to determine the occurrence and quality of both sleep and waking [27]. Sleep electrocorticogram (ECoG) was used to evaluate the effect of light-exposure on sleep in these studies. Unfortunately, we do not have the equipment needed for evaluating sleep in rats. We speculate that light-exposure induce wakefulness and alertness, and that the amplitude of delta activity in ECoG, a marker of sleep, is reduced in the continuous light rats compared to the controls, but further research would be needed to confirm this speculation. The potential limitations of our study should be noted and we would like to pursue this further when we have the capacity to perform EcoG. In addition, our study missed sham+light or sham+melatonin group and we plan to replicate this study with shoes added controls in the future.
Taken together, we observed that melatonin can ameliorate aberrant upregulation of HDACs and histologic indicators of cardiac hypertrophy. Melatonin may be useful for the treatment of cardiac hypertrophy and heart failure in juveniles.
Figures and Tables
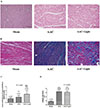 | Fig. 1Effects of AAC on the cardio fibrosis under different light conditions.Representative myocardial HE (A) staining and Masson collagen staining (B). Effects of chronically increased pressure overload on the cross-sectional area of myocardial fibers (C) and collagen deposition (D) under different light conditions. **p<0.01, ***p<0.001 vs. sham group; ###p<0.001 vs. AAC group. Images were acquired at 200×magnification. “n” represents the number of cardiac fibers in Fig. 1C, and the number of image fields in Fig. 1D.
|
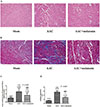 | Fig. 2Effects of AAC on the cardio fibrosis in melatonin-treated rats.Representative myocardial HE (A) staining and Masson collagen staining (B). Effects of chronically increased pressure overload on the cross-sectional area of myocardial fibers (C) and collagen deposition (D) in melatonin-or vehicle-treated rats. *p<0.05, ***p<0.001 vs. sham group; ###p<0.001 vs. AAC group. Images were acquired at 200×magnification. “n” represents the number of the cardiac fibers in Fig. 2C, and the number of image fields in Fig. 2D.
|
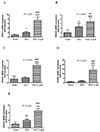 | Fig. 3(A-E) Relative HDAC1, 2, 3, 4 and 6 mRNA expression levels in ventricular cardiomyocytes of juvenile rat under different light exposure (real-time PCR).
*p<0.05, **p<0.01, ***p<0.001 vs. sham group; ##p<0.01, ###p<0.001, vs. AAC group; all n=8.
|
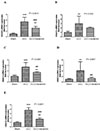 | Fig. 4(A-E) Relative HDAC1, 2, 3, 4 and 6 mRNA expression levels in placebo-treated or melatonin-treated juvenile rat ventricular cardiomyocytes (real-time PCR).
**p<0.01, ***p<0.001 vs. Sham group; ##p<0.01, ###p<0.001, vs. AAC group; all n=8.
|
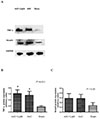 | Fig. 5Relative TNF-α and Smad4 protein expression levels in AAC-treated juvenile rat ventricular cardiomyocytes (Western blot).Western blot images (A) and relative quantification of TNF-α (B) and Smad-4 (C) protein expression in juvenile rat ventricular cardiomyocytes. *p<0.05 vs. sham group, all n=4.
|
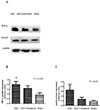 | Fig. 6Relative TNF-α and Smad4 protein expression levels in AAC-treated juvenile rat ventricular cardiomyocytes (Western blot).Western blot images (A) and relative quantification of TNF-α (B) and Smad-4 (C) protein expression in juvenile rat ventricular cardiomyocytes. *p<0.05 vs. sham group, all n=6.
|
Notes
Author contributions Y.W. made the study design and wrote the first draft of the manuscript; F.S. and J.Z. completed the analysis and interpretation of data; L.L. and F.J. built the animal modles; K.J. performed the echo; Q.Y. decided to submit the paper for publication. All authors had seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript.
References
1. Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, Ortiz GG, Acuña-Castroviejo D. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995; 18:1–11.

2. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002; 2:181–197.

3. Simko F, Pechanova O, Pelouch V, Krajcirovicova K, Celec P, Palffy R, Bednarova K, Vrankova S, Adamcova M, Paulis L. Continuous light and L-NAME-induced left ventricular remodelling: different protection with melatonin and captopril. J Hypertens. 2010; 28:Suppl 1. S13–S18.

4. Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, Kamari Y, Shen-Orr Z, Zisapel N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006; 119:898–902.

5. Ferguson BS, McKinsey TA. Non-sirtuin histone deacetylases in the control of cardiac aging. J Mol Cell Cardiol. 2015; 83:14–20.

6. Schuetze KB, McKinsey TA, Long CS. Targeting cardiac fibroblasts to treat fibrosis of the heart: focus on HDACs. J Mol Cell Cardiol. 2014; 70:100–107.

7. Gallo P, Latronico MV, Gallo P, Grimaldi S, Borgia F, Todaro M, Jones P, Gallinari P, De Francesco R, Ciliberto G, Steinkühler C, Esposito G, Condorelli G. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res. 2008; 80:416–424.

8. Kang SH, Seok YM, Song MJ, Lee HA, Kurz T, Kim I. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol Pharmacol. 2015; 87:782–791.

9. Wu TH, Kuo HC, Lin IC, Chien SJ, Huang LT, Tain YL. Melatonin prevents neonatal dexamethasone induced programmed hypertension: histone deacetylase inhibition. J Steroid Biochem Mol Biol. 2014; 144:253–259.

10. Hwang B, Qu TY, Hu CT, Chen HI. Hemodynamic and neurohumoral changes after abdominal aortic constriction in rats. Proc Natl Sci Counc Repub China B. 1999; 23:149–157.
11. Şehirli AÖ, Koyun D, Tetik Ş, Özsavcı D, Yiğiner Ö, Çetinel Ş, Tok OE, Kaya Z, Akkiprik M, Kılıç E, Şener G. Melatonin protects against ischemic heart failure in rats. J Pineal Res. 2013; 55:138–148.

12. Zhang S, Li H, Yang SJ. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacol Sin. 2010; 31:671–678.

13. Kang S, Liu Y, Sun D, Zhou C, Liu A, Xu C, Hao Y, Li D, Yan C, Sun H. Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One. 2012; 7:e48185.

14. Williams SM, Golden-Mason L, Ferguson BS, Schuetze KB, Cavasin MA, Demos-Davies K, Yeager ME, Stenmark KR, McKinsey TA. Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol. 2014; 67:112–125.

15. Mizrak B, Parlakpinar H, Acet A, Turkoz Y. Effects of pinealectomy and exogenous melatonin on rat hearts. Acta Histochem. 2004; 106:29–36.
16. Lee E, Song MJ, Lee HA, Kang SH, Kim M, Yang EK, Lee DY, Ro S, Cho JM, Kim I. Histone deacetylase inhibitor, CG200745, attenuates cardiac hypertrophy and fibrosis in DOCA-induced hypertensive rats. Korean J Physiol Pharmacol. 2016; 20:477–485.

17. Lochner A, Huisamen B, Nduhirabandi F. Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front Biosci (Elite Ed). 2013; 5:305–315.
18. Lkhagva B, Lin YK, Kao YH, Chazo TF, Chung CC, Chen SA, Chen YJ. Novel histone deacetylase inhibitor modulates cardiac peroxisome proliferator-activated receptors and inflammatory cytokines in heart failure. Pharmacology. 2015; 96:184–191.

19. Hohl M, Wagner M, Reil JC, Müller SA, Tauchnitz M, Zimmer AM, Lehmann LH, Thiel G, Böhm M, Backs J, Maack C. HDAC4 controls histone methylation in response to elevated cardiac load. J Clin Invest. 2013; 123:1359–1370.

20. Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell. 2008; 19:4141–4153.

21. Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med. 2007; 13:324–331.
22. Korkmaz A, Rosales-Corral S, Reiter RJ. Gene regulation by melatonin linked to epigenetic phenomena. Gene. 2012; 503:1–11.

23. Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002; 53:822–830.

24. Gupta S, Tripathi CD. Current status of TNF blocking therapy in heart failure. Indian J Med Sci. 2005; 59:363–366.

25. Shaki F, Ashari S, Ahangar N. Melatonin can attenuate ciprofloxacin induced nephrotoxicity: Involvement of nitric oxide and TNF-α. Biomed Pharmacother. 2016; 84:1172–1178.

26. Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999; 31:667–678.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download